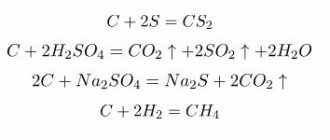The hardest stone in the world is one of the modifications of carbon, diamond, the formula of which is denoted by the Latin letter C. This is a simple substance with a crystalline face-centered cubic lattice. It conducts electricity poorly, is stable at high pressure and does not melt at natural temperatures. Among the main physical properties of a diamond, high density, fragility, transparency and incredible shine should be noted.
Diamond: what is it and how did it appear?
Very little is known about the history of the origin of the diamond. Scientists consider the mineral diamond to be the same age as our planet. It was formed several million years ago by high pressure and temperature. The structure of the mineral is represented by carbon atoms, which we encounter every day in everyday life (pencil leads are made of graphite, which also consists of pure carbon).
In nature, diamonds exist in the form of small crystals, which rarely reach the size of a bird's egg.
Fossil and mine diamonds are stones that have an extremely dense structure and, in their pure form, are harder than any substance known today.
I wonder what word the word diamond comes from.
The origin of the word diamond has a long history. The ancient Romans and Greeks called it Adamas, meaning "solid" or "invincible".
Already from the Greek “Adamas” came the Arabic word “Almas”, which in Russian turned into the familiar “Almaz” to our ears.
In English, a diamond is called Diamond, and in Slavic languages these minerals are called diamonds.
Diamond jewelry
The modern jewelry market offers a variety of jewelry with diamonds of different sizes.
A wedding ring with diamonds will make the bride and groom stand out, because expensive jewelry will indicate their financial situation and status.
A set of jewelry completely covered with small diamonds will subtly indicate the wealth of a wealthy woman over the age of 40.
A legendary stone with a long history
Diamonds were first talked about as precious stones more than 5 thousand years ago, when the people of India noticed shiny stones that could not be broken, but could easily be used to process other minerals.
Natural diamond attracted the ancient inhabitants of India with its transparency and brilliance, gradually becoming a kind of hard money for which they could purchase food, clothing and other valuables. Numerous rulers of the country did not subject these stones to any processing, but kept them in their entirety, placing them in treasuries.
In Europe, they learned about the existence of diamond crystals only in the era of Alexander the Great, who organized a campaign against India in order to capture these precious stones.
Only in the Middle Ages did craftsmen in the Belgian city of Bruges learn to process these minerals, turning them into diamonds.
Gemstone with strong shine
Diamond gemology classifies this crystal as a semi-precious stone. She studies its chemical composition, physical and optical properties, as well as decorative and artistic characteristics.
Diamonds are crystals that have a natural shine. On the Mohs comparative density scale they have a maximum value of 10 and are suitable for processing any other minerals.
Most often, colorless transparent minerals are found, but occasionally they can have pink, bluish, gray, yellow and even greenish shades. This is due to the fact that, under the influence of high temperature and pressure, carbon enters into chemical reactions with other substances, changing the structure of the diamond crystal lattice.
Most often it is mined in rocks located at a depth of 600 meters or more. Deposits rarely come to the surface and can be mined simply by sifting the soil.
Classification by chemical composition
In its pure form, diamond is extremely rare in nature. The bulk of crystals have certain flaws associated with the presence of “foreign” boron or nitrogen atoms, or with the absence of individual fragments of the crystal lattice.
Depending on the structure of the crystal lattice, the type and class of this mineral are distinguished:
- the most common type I in nature with individual nitrogen atoms present;
- having a yellowish tint, type IA, which contains groups of nitrogen atoms;
- the extremely rare type IB, in which the nitrogen content is minimal, and the stones are orange, brown and even green;
- type II, containing virtually no nitrogen;
- pure or 100% Type IIA diamonds contain no nitrogen at all;
- Type IIB diamonds, which are blue or gray in color, contain boron in varying concentrations.
Previously, the description of mined minerals was carried out by eye, but now special equipment is used for these purposes.
Types of diamonds
There are several classifications of gemstones. According to the first, there are the following types of diamonds:
- Diamond - shining samples processed by a master.
- Board - irregular fine-grained specimens. They are black in color and even more durable than regular diamonds.
- Ballas are spherical gems with a radiant structure. They are distinguished by a cloudy grayish structure, for which they are nicknamed translucent crystals.
- Carbonado - crystals of black, gray shades, dense or coarse-grained structure.
- Yakutite is a dark mineral with inclusions and a silvery sheen on the surface.
In addition to this division into types, all diamonds after extraction are divided depending on size:
- Small minerals include minerals whose weight does not reach one third of a carat.
- Medium stones are those that do not exceed a weight of 1 carat, but are not small.
- Large diamonds are gems larger than one carat.
Mining a diamond and turning it into a diamond
Diamond is a mineral with semi-precious characteristics. Today, its significance in the world is somewhat different from the medieval one, since the stone is used in industry for geological exploration and drilling. No rock will be an obstacle to creating industrial drills based on diamond crystals, and prospectors can easily obtain information about the structure of the earth’s crust.
To extract the mineral, it is necessary to find a kimberlite pipe, through which in ancient times there was a deep eruption of substances contained in the earth's core. From the rock contained in it, a diamond of the highest quality is mined, which, after being examined for color and the presence of cracks, is classified.
The most elite diamond is considered to be one that does not have any inclusions of foreign matter. It is sent for cutting, after which it turns into a diamond sparkling in the sun with its many facets.
Medicinal properties
Diamonds are not only valued for their beauty and brilliance, but also have beneficial and healing properties. In folk medicine, the gem is used as “color therapy”. The method of treatment with color is in the direction of glow from ultraviolet rays on a person, which cleanses the body of diseases.
Another treatment method in lithotherapy is to immerse the diamond in water for several days and drink it. The resulting concentrate tones the human body, killing microbes and forcing the body's processes to work in the right direction.
Traditional healers often use the mineral in treatment, believing in the natural power of the stone. The following are the pathologies for which diamond therapy is used:
- asthma;
- kidney failure and kidney stones;
- accelerates the metabolic process;
- hepatitis;
- bronchitis;
- prostate diseases;
- joint pathologies;
- skin diseases.
What do diamonds look like?
Despite popular belief among ordinary people, a real diamond found in a rock is very different from a cut diamond. It has virtually no natural shine and acquires it only after treatment with water. A non-specialist can easily pass by such a mineral, without even suspecting that a real treasure lies in front of him.
Very often there are defects in the structure of the crystal, which, upon impact, can crumble into dozens of fragments.
The unique structure of diamond determines its color, transparency, luster and almost complete absence of electrical conductivity.
The density of diamond is significantly higher than that of graphite, although their chemical classification is exactly the same. The molar mass of either substance is 12 grams per mole, but the structure of diamond is much denser than graphite.
Artificial analogues of diamonds
Thanks to research, it was possible to discover a method for synthesizing agglomerate, thanks to which it is possible to create stones identical in structure to natural diamonds. Synthetic specimens - cubic zirconia - are close in their characteristics to real crystals. The creation of synthetic diamonds is a necessary measure, since the existing reserves in the deposits are not enough to provide all areas of industry with a large number of stones. Despite the efforts of scientists, there are minor differences in the appearance and structure of diamonds and cubic zirconia.
Natural and synthetic diamonds are very similar, but not identical.
Molecules with strong chemical bonds
The chemical composition of diamonds is represented by ordinary carbon, but the unusual molecular structure gives the substance properties that are characteristic only of it.
According to the periodic table, the mineral is designated C (carbon), just like its related graphite. Diamonds are made up of carbon atoms that are arranged at the vertices of a complex structure. The crystal lattice of diamond is face-centered, cubic. It makes the mineral extremely resistant to external influences.
The molecules that make up diamond are cube-shaped crystals, the vertices of which are fixed by carbon atoms. Inside the crystal there are 4 more carbon atoms that have a chemical bond with the vertices of such a cube. Such structural features provide a peculiar movement of electrons of carbon atoms, and the crystal lattice becomes unusually strong.
Silicon, low-temperature modification of tin and some other simple substances have a similar molecular and non-molecular structure.
Deep diamonds are formed from organic carbon
Rice.
1. Diamond crystal in kimberlite. Specimen from the Field Museum of Natural History in Chicago. Photo from flickr.com
Australian scientists, based on the results of isotope studies, have concluded that the carbon that makes up diamonds formed in the transition zone of the mantle is of organic origin. The authors suggest that the primary source of carbon for deep-seated diamonds was marine sediments enriched in organic matter, pulled into the mantle by subducting lithospheric plates. At a depth of more than 400 kilometers, these sediments were processed, and the carbon they contained became part of the diamonds, which were then carried to the surface by plumes - ascending flows of mantle matter.
Most diamonds are confined to explosion tubes—vertical structures formed when gas-enriched mantle melt breaks through the earth’s crust. The igneous rocks that fill the explosion tubes - kimberlites, lamproites and lamprophyres - contain a large number of xenoliths (altered fragments of mantle rocks) - peridotites and eclogites - to which diamonds are confined.
In tectonic terms, diamond-bearing explosion pipes are located in zones of ancient continental crust located above large areas of the mantle with abnormally low velocities of transverse (or shear) seismic waves - low-shear-velocity provinces (LLSVP), which are still are called superplumes (for more information about superplumes, see the news Supercontinental cycles are synchronized with periods of superplume activity, “Elements”, 01/10/2020). Branches of superplumes—mantle plumes—which are also characterized by reduced velocities of transverse seismic waves, rise up thousands of kilometers, reaching the lower boundary of the lithosphere. Here, as a result of a drop in pressure, a gas phase is released from the mantle melt, and boiling magma breaks through to the surface along weakened zones, forming explosion tubes.
Mineral and gas-liquid inclusions in diamonds indicate that the vast majority of them formed in the upper mantle at a depth of 150 to 300 km, and were then brought to the surface as part of mantle xenoliths. But among them there is a small group - about one percent of all diamonds found - which, judging by the composition of the inclusions, were formed deep in the bowels of the Earth, in the transition zone of the mantle, located at a depth of 410 to 660 km. The former are conventionally called lithospheric, and the latter - sublithospheric (or ultra-deep continental).
An even smaller proportion (substantially less than one percent of the total) of diamonds is found in ancient oceanic lithosphere, preserved in ophiolite complexes around the world. Accessory diamonds in peridotites of ophiolite belts are not of commercial value due to their tiny size and low quality, but are extremely important from a scientific point of view. Previously, scientists proved that such oceanic diamonds were formed at significant depths, and the presence of hydrocarbons in their gas-liquid inclusions indicates that at these depths the melt already contained carbon, which served as a source of diamond matter (see news Diamonds in peridotites were formed from liquid and gaseous hydrocarbons of the transition zone of the mantle, "Elements", 02.11.2017).
Rice. 2.
Carbon isotope composition of diamonds of three genetic groups (
a
- lithospheric continental;
b
- ultra-deep continental;
c
- oceanic), expressed in terms of δ13C (in ppm).
δ13C is the deviation of the 13C/12C isotopic ratio from the signature of the PDB standard sample, the Cretaceous belemnite Belemnitella americana
from the Peedee Formation in South Carolina.
in pink
;
green
—peridotite diamonds;
in various shades of orange and white
- ultra-deep diamonds from the deposits of Juina-5 (Brazil), Jagersfontein and Monastery (South Africa), Sao Luis (Brazil) and Kankan (Guinea), as well as single ultra-large diamonds of the CLIPPIR class (cores - crystal cores; rims - edge zones);
in various shades of blue
- oceanic diamonds from the Rai-Iz (Polar Urals), Mirdirta (Albania) and Pozanti-Karsanti (Turkey) deposits.
Gray vertical bars
are isotopic ranges: organic carbon, mantle carbon, and carbonate carbon.
Figure from the discussed article in Scientific Reports
Australian geologists from the School of Earth and Planetary Sciences at Curtin University in Perth decided to use isotopic data to determine the source of carbon that is part of diamonds of all three groups. The results showed that lithospheric, ultra-deep continental and deep ocean diamonds are characterized by different ratios of organic and inorganic carbon in their composition. At the same time, surprisingly, the largest amount of organic carbon was found in two deep varieties (Fig. 2).
If the connection of continental diamonds with plume magmatism is obvious, then to explain the appearance of deep diamonds in the oceanic crust formed from the material of the upper mantle, different mechanisms were proposed at different times, such as the return flow of mantle material that occurs when the subducting plate subsides (S. Arai, 2013 Conversion of low-pressure chromitites to ultrahigh-pressure chromitites by deep recycling: A good inference) or mantle upwelling (J. Yang et al., 2015. Diamond-bearing ophiolites and their geological occurrence).
The authors of the study under discussion put forward their own alternative hypothesis. Using examples of specific ophiolite belts, they showed that diamonds in them are associated not with classical ophiolite sequences, which represent normal oceanic lithosphere formed at mid-ocean ridges, but with superimposed derivatives of large igneous provinces (see Large igneous province, LIP) or basalts of ocean islands (see Ocean island basalt, OIB). The former, as is known, are formed above superplumes, and the latter are characteristic of so-called hot spots, which are a projection onto the surface of ascending mantle plumes (for more information about large igneous provinces, see the news. In large igneous provinces there could be two sources of magma, “Elements”, 18.04. 2018).
To confirm their hypothesis about the connection between oceanic diamonds and plumes, the researchers cite the findings of diamonds in two places in the modern ocean - in the Hawaiian Islands and off the island of Malaita in the southwestern part of the Ontong Java oceanic plateau. Both of these points are associated with mantle plumes. Diamonds here are confined to mantle xenoliths, where they occur together with ultrahigh-pressure minerals. Their δ13C isotopic values are very low—from −30 to −20‰, that is, the same as for ancient oceanic diamonds from ophiolite belts.
Interestingly, the ages of the known diamondiferous ophiolites (430, 395, 165, 125 and 95 Ma) generally coincide with the peaks of oceanic mantle plume activity over the past 500 Ma. A similar correlation can be traced between continental plumes and diamondiferous kimberlites (Fig. 3).
Rice. 3.
Peaks of activity of mantle plumes - oceanic (shown
in blue
) and continental (shown
in orange
).
The vertical axis
is the number of events,
the horizontal axis
is the age in million years.
The dotted line
is the temporary distribution of diamond-bearing kimberlites,
purple diamonds
are finds of oceanic diamonds,
purple diamonds with a white border
are finds of ultra-deep continental diamonds, including the largest diamond in the world, the Cullinan.
Figure from the discussed article in Scientific Reports
The authors associate the periodicity of plume activity with global mantle dynamics caused by the supercontinental cycle.
Separately, the authors dwell on the extreme heterogeneity of the isotopic parameters of sublithospheric diamonds. The δ13C values in them range from −30 to +3‰ (Fig. 2). At first glance, with such a scatter, it is impossible to definitely talk about any one source of carbon. However, a detailed study of diamonds from the Brazilian Juina 5 and Sao Luis deposits, as well as fragments of several large diamonds of the CLIPPIR class (this abbreviation stands for Cullinan-like tends to be large, inclusion-poor, relatively pure, irregularly shaped, and resorbed - “ similar to “Cullinan”, large, practically without inclusions, relatively pure, irregular in shape and with traces of resorption”; resorption is the name given to processes that change the shape of a crystal after completion of its growth, for example, dissolution) showed that all of them demonstrate isotopic zoning with very light organic fractions in the core (δ13C from −28 to −20‰) and heavier mantle carbon isotope composition at the edges (δ13C from −15 to −5‰). The only exception is the diamonds from the Kankan deposit with abnormally heavy carbon, for which the authors have not yet found an explanation.
Based on the fact that the cores of ultra-deep continental diamonds have the same isotopic composition as oceanic diamonds, and also on the fact that both are confined to places of plume magmatism, the authors suggest that diamonds of these two groups have a common origin. They were formed from the same organic carbon drawn into the transition zone of the mantle by a subducting plate, and then were carried to the foot of the lithosphere by mantle plumes.
In the case of thin oceanic lithosphere, plumes approach close to the surface, and virtually unchanged deep-seated diamonds are found in xenoliths of hot spots above the plumes. If the mantle plume abuts the thick continental lithosphere, diamonds continue to grow in magma chambers of the upper mantle, where a shell of heavy carbon grows on a core that is lighter in isotopic composition (Fig. 4).
Rice. 4.
Model of the origin of three types of diamonds.
Oceanic and ultradeep continental diamonds (cores only) form in the mantle transition zone from subducted organic carbon and are then transported to the base of the lithosphere by mantle plumes. Lithospheric diamonds form in the continental lithosphere. Here, outer rims grow on the cores of sublithospheric diamonds. Orange and dark green
show the continental lithosphere (crust and upper mantle);
blue
- oceanic lithosphere (crust and upper mantle), plunging into the mantle in the subduction zone;
yellow
—mantle transition zone;
red
- mantle plumes;
black diamonds
are diamonds formed in the transition zone of the mantle;
white diamonds
are diamonds formed in the lithosphere;
black diamonds with a white border
are diamonds of mixed origin.
Figure from the discussed article in Scientific Reports
This is the first model that links all three types of diamonds to a single process of rising mantle plumes. It also explains well why oceanic diamonds are always very small (micro- or nanometer-sized): in fact, these are the cores of ultra-deep diamonds without a “lithospheric” shell. Also, for the first time, scientists have proven that carbon of primary organic origin played the main role in the formation of deep-seated diamonds.
Source:
Luc S. Doucet, Zheng-Xiang Li, Hamed Gamal El Dien.
Oceanic and super-deep continental diamonds share a transition zone origin and mantle plume transportation / Scientific Reports
. 2021. DOI: 10.1038/s41598-021-96286-8.
Vladislav Strekopytov
Artificial stones and their use
Today, scientists have learned to create artificial diamonds in laboratory and industrial conditions. Under the influence of high temperatures and pressure, they are formed and consist of carbon, and their unique composition depends on the substances used in the production process.
Synthetic diamonds are most often used to make tools that can cut and polish other minerals, as well as fully cut natural stones, turning them into diamonds.
The characteristics and types of natural stones directly depend on their chemical composition, as well as the presence of nitrogen and boron atoms in natural formations.
Jewelers divide these stones into several classes. The characteristics of a mineral are determined by purity, color and size. Diamonds come in a variety of colors and shades, and in most cases their evaluation is carried out after cutting.
Cutting methods
The types of diamonds and their names most often depend on their transparency. Specialists first carefully study the mineral, removing even the slightest cracks and defects that can be found on the stone. Only after this they begin to process it.
The most commonly produced diamonds are semi-regular and rhombic dodecahedron. This is a dodecahedron. consisting of identical rhombuses and having 14 vertices.
This type of cut is suitable for all types of diamonds, even if they are translucent. Other types of cutting are very rare in the world, which are associated with the desire to preserve as much as possible the size of a diamond mined in natural conditions.
Due to the high density, cutting tools are used for cutting, the cutting parts of which are made of the same material, and the edges are polished using diamond dust, as a rule. of artificial origin.
Diamond processing
Before processing a diamond, the jeweler selects the best material without significant damage or natural defects. If there are microcracks on the stones, they are removed or masked using special devices that change the structure using environmental indicators.
After this, cutting is carried out. The most popular diamond shapes are hexagonal. This type of cut is considered universal, as it is suitable for diamonds regardless of the type, color and natural characteristics of the specimens. Moreover, this form allows you to preserve the natural size of large and medium-sized gems and not reduce the size of small crystals even further.
Colors and shades of processed stones
Next we will tell you what color a diamond is. The color range of this mineral in natural conditions is not very diverse. The most common diamond is a transparent one that has no color inclusions.
Colored diamonds are much less common in the natural environment.
It is extremely rare that the color of a diamond can be blue, grey, pink, yellow or brown. There are only a few known deposits in the world where blue and black minerals are mined, which most likely are of cosmic origin and fell to Earth along with meteorites.
If we talk about what color a diamond is, then the situation here is somewhat different. Thanks to the large amount of artificial stone, jewelers can give them different shades of color. There are diamonds that differ very significantly in color from natural stones.
If previously the color of a precious item depended on the color of the diamond, in recent years the public has been shown a brown stone and introduced to a fiery diamond.
DIAMOND COLORS
For a gemologist, the ideal diamond is a transparent, completely colorless crystal.
The color of stones is associated, as experts put it, “with the presence of intrinsic or impurity crystal lattice defects in the structure.”
The color palette of these precious stones is large:
- black;
- white;
- yellow;
- brown;
- blue;
- red;
- blue.
Diamond colors
The choice of fancy colors is large enough to find a stone to your liking and budget.
To successfully invest in diamonds, don’t be lazy to fly to the USA (closer to Israel, Tokyo, Botswana, Hong Kong). Independent gemological laboratories are located in these countries. It is there that they can issue a certificate for the purchased diamond.
We recommend: OPAL - a natural rainbow of gems, interesting legends and myths
The GIA Institute is the most authoritative gemological organization in the world; its certificates are not discussed or questioned.
Yellow Diamond
- Occurs most often.
- It gets its color from nitrogen impurities.
- The complex physical chemistry of the crystal will show how many impurities are in the stone and how they are located.
- There is a rare group of yellow diamonds whose color is given by nickel.
- Previously it was believed that “nickel” yellow adamant could only be synthetic.
Yellow Diamond
Blue Diamond
Blue and light blue diamonds are divided into 2 categories.
- Boron gives one shade of the sky and sea; these amazing stones have the property of conducting electric current - “hole conductivity”.
- The second type of stones does not contain boron; Based on the results of spectrography, it was decided that hydrogen could impart color. There are refined stones that are given color. Yellow diamonds can be used to make blue diamonds - there are special technologies for this. But it will be impossible for a non-specialist to determine by any signs, by color, whether it is a natural diamond or whether its noble features have been taken on by an ennobled stone.
Blue Diamond
Blue Diamond
A diamond stone of heavenly color - a drop of the sea or a piece of the sky...
- Blue diamonds are rare and beautiful. There should be no gray color in the blue of the stone.
- A rich, thick color will greatly increase the price you will have to pay for the jewelry.
- The chemistry of the stone is simple - the crystal gets the color of the sky from boron.
The most famous and expensive blue diamond is the Hope Diamond, weighing 44.40 carats. Estimated cost: 200-250 million US dollars. Exhibited at the Museum of Natural History (Washington State, USA).
Blue Diamond
Green Diamond
Green cut crystals are the rarest among their fancy-colored counterparts.
- The color of a diamond comes from radioactive radiation; under the influence of α- and β-particles, the diamond acquires a green color, but not completely - the stone seems to be covered with green skin. This is due to the low penetrating ability of this type of radiation.
- Possible green partial pigmentation in uneven spots. This is why many green diamonds are mined, but only a few are cut.
- Specimens that were exposed to long-term γ- and neutron radiation during growth are characterized by high penetrating ability). These very rare stones have a rich green color.
Green Diamond
The largest green diamond is the Green Dresden, weighing 40.70 carats.
Red diamond
Not every jeweler can boast of having seen a red diamond.
- The color of red and pink crystals is not given by impurities - the shade depends on the plastic deformation of the stone during the growth process (according to gemologists).
The largest red diamond is Muzaya Red Diamond. Its weight is 5.11 carats. This treasure is worth 20 million US dollars.
Not all treasure owners are eager to share their joy from acquiring a piece of jewelry. Therefore, information about diamonds of fabulous value is leaked to the media reluctantly.
Red diamond
Physicochemical properties of the mineral
The unique properties of diamond have made this mineral extremely popular not only as a precious stone. The optical properties and ability of crystals to refract light make them necessary in scientific research, and the ability to create virtually dull-resistant medical instruments increases their demand in surgery.
The physical and chemical properties of a mineral directly depend on the impurities it contains. Diamond is a simple substance, the basis of which is carbon atoms connected in a face-centered crystal. The specific gravity of diamonds is 12 grams per mole, but the presence of impurities can slightly change this figure.
Next, we will tell you what properties a diamond has and what has made this mineral extremely popular nowadays not only as jewelry.
AREAS OF USE
Application in science and technology
Due to its unique properties and features, diamond is used:
- in medicine - for the manufacture of instruments for microsurgery,
- in drilling technology - for the production of heavy-duty drills,
- in the production of tools - for processing metals and alloys.
We recommend: How is the purity of an emerald determined?
High-pressure apparatus, the stone-cutting industry, electronic devices, physics - all this will simply stop without a unique stone.
The magical properties of diamond
- People have accumulated a lot of observations, beliefs, and superstitions associated with amazing stones.
- The Indians were the first to understand the beauty of the stone, define its magic, and consider the diamond a symbol of the purity of the soul.
- The properties of diamonds were described and used by astrologers, priests, sages, scientists...
Here are a few of the properties attributed to stones of amazing beauty and strength:
- Strengthens strength, makes the properties inherent in the owner stronger and brighter. It should be noted that all properties are both good and bad.
- Protects against the evil eye, astral attacks, and evil witchcraft.
- It will cleanse the room from negative energy, from larvae, from any evil entities. Provided there are no other gems in the room.
- A stone worn on the left hand will help defeat enemies.
- Protects pregnant women and women in labor.
- Worn by a criminal, the stone will act against the owner.
Adamant will only work if it was given to the owner as a gift.
A stone bought for oneself, stolen, or dubiously obtained resists fiercely.
Who is suitable according to their zodiac sign?
Suitable for everyone, if only you had the money to buy it. Diamond in the horoscope is patronized by the Sun and Venus. Since the sun is present in every person's natal chart, this sparkling miracle is suitable for everyone (but with reservations).
- Adamant's first favorites are Leos. They, the kings of the horoscope, should rightfully wear the royal stone.
- Aries, in whose veins molten fire flows, will be successfully helped in life by a red or pink diamond.
The astrological meaning of a diamond is a combination of natural elements, Earth and Water, Fire and Air.
Indian astrologers believe that diamond jewelry should be worn for the first time on Friday, the day of Venus.
Hard stone with high transparency
Ideal stones, which contain no impurities, are completely transparent and have a very high refractive index of light by the diamond.
They perfectly refract light, dividing it into all the colors of the rainbow, which is very valuable in jewelry production. The refractive index of diamond ranges from 2.417 to 2.421 units, and the dispersion coefficient is 0.0574.
The more the angle of light is refracted, the better the diamond sparkles and plays with colors.
The refraction of light allows a diamond to change its speed better than quartz, redirecting it to another medium.
Diamonds are often used to demonstrate the optical characteristics of light and show how it splits into wavelength-dependent color fragments.
According to their physical properties, diamonds are divided into transparent, translucent and opaque. They can have a diamond greasy shine and density. One cubic centimeter of stone can have a mass of 3.5 to 3.53 grams, and its hardness reaches a maximum of 10 on the Mohs scale.
Diamond use
Reference. Diamond is widely used in human life. In jewelry, a diamond is a diamond, jewelry with it is very valuable. In the industrial sector, small pebbles or artificially produced stones are used. Diamond chips make it possible to provide any tool with high productivity and high cutting quality. These could be scissors for cutting metal, glass, or materials used in grinding. Working speed and efficiency increase dramatically when using diamond.
“Diamonds are a girl’s best friend...”, indeed, the lyrics from the song perfectly describe the main purpose of a diamond. The mineral is very attractive, with a special shine and glow that attracts attention. Jewelry with it is incredibly sophisticated and looks very expensive. The best gift for any person who appreciates the quality of precious stones. In addition, the stone has magical and healing properties and is suitable for many people, but only with a pure and sincere soul. After all, only diamond has transparency and great hardness. The mineral differs in its deposit from other natural substances. Diamond is mined both inside the earth and “extraterrestrially”, which are formed by meteorites.
Has maximum thermal conductivity
These basic physical properties of the mineral make it extremely popular in industry, science and medicine, where they are used in the manufacture of complex instruments and optical devices.
Minerals are fragile and have extremely high thermal conductivity. At room temperature, their thermal conductivity is 4 times higher than that of copper.
The high refractive index of crystals makes it possible to create instruments for scientific research, convert the energy of sunlight into electricity, and also produce semiconductors capable of operating at temperatures up to 100 degrees Celsius.
Diamond melts at a temperature of 3700-4000 degrees and burns at 850 degrees Celsius. When burned, the mineral glows blue, yellow and green, but doing this is extremely expensive and unprofitable.
The chemical properties of diamonds depend on the mechanical inclusions it contains.
From a chemical point of view, this stone is ordinary carbon, having the same atomic composition as graphite.
Diamond in chemistry is an isomorphic substance, which, under certain conditions, may include nitrogen, boron, as well as aluminum and silicon.
Most natural diamonds contain nitrogen atoms that replace the vertices of the face-centered crystals. Nitrogen-containing minerals are characterized by high luminescent properties, as well as the ability to scatter X-rays.
Natural stone is an excellent dielectric and does not dissolve in acidic or alkaline environments. In the production of artificial crystals, aluminum and silicon are introduced into their composition, which give them distinctive characteristic features that are in demand in a particular field.
What are diamonds used for?
As mentioned above, the traditional use of diamonds for cutting and producing diamonds is gradually losing its relevance. The physical and chemical properties of this mineral have made it extremely popular in various areas of modern life.
The stone is very hard, is an excellent dielectric and has high thermal conductivity. On this basis, the use of diamond has become widespread in industry.
Using diamond tools, high-precision cutting of any materials is carried out. Special crowns made of this material are equipped with drilling rigs that conduct geological exploration and drill holes in mines and quarries.
Diamond lubricant has an extremely low coefficient of friction on metal (0.1) due to the formation of films of adsorbed gas, and the stones themselves adhere perfectly to fat mixtures. It is used to lubricate various parts and tools, which significantly increase their working life and are less likely to fail.
Origin of the diamond
The main diamond deposits are located in:
- Africa;
- Russia;
- Australia;
- Canada.
Since mining began long before research into the crystal, natural treasures have been significantly depleted. This is reflected in both the price and quality of the extracted material. However, Brazilian and South African deposits, discovered only a century and a half ago, continue to provide humanity with rare, but very elegant gems. The origin of each stone affects its physical and chemical properties , as well as its price and appearance.
Modern industrial production
In ancient times, diamonds were found in rock falls in the Himalayas (India), but this mining method is outdated and unprofitable.
Today, new quarries and mines are being opened to search for natural stones.
Due to the fact that this mineral was formed deep in the earth’s crust, the main depth of the deposits is 600 meters or more.
Geologists conduct exploration of the area and find kimberlite pipes that may contain these semi-precious stones.
The earth is removed using a mine or open pit method to the required depth, and for hard rocks, a directed explosion is used.
When a kimberlite pipe is reached, tunnels are laid in different directions, and the rock is lifted for processing. Typically, to obtain 1 carat of diamonds, it is necessary to process several hundred tons of all rocks mined in a mine or quarry.