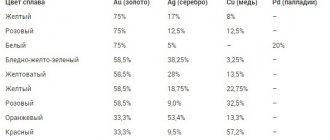
Metals in jewelry alloys
TIN
If there is a lot of tin in the alloy, then the output is matte shades of gray. Such alloys are light, but not strong and brittle. Products of Chinese origin demonstrate similar quality.
COPPER
When copper predominates in the alloy, the color turns out to be more brown, and the metal has an antique effect. A large presence of copper is typical for Czech jewelry. When buying jewelry, do not forget that for copper compounds, oxidation in air is a natural process. Copper products darken over time, so they need to be cleaned regularly. Methods for cleaning copper products include rubbing the surface with a soft cloth until polished. Never use solutions containing cleaning agents.
BRASS
Similarly, brass is used, which is essentially a copper-based alloy. Products made of brass have yellowish-green shades with an admixture of gray. The color is somewhat similar to bronze, but may vary due to other components. Brass has found application in the manufacture of decorative elements in the antique style by Czech manufacturers.
ALUMINUM
Aluminum is also favored by jewelry manufacturers from the Czech Republic. It is close in color to steel, just as glossy and shiny. Steel added to alloys increases strength. Used in the production of fashion jewelry.
NICKEL
Nickel is an almost black metal. Easily oxidized. When contained in large proportions in the alloy, it can cause allergies. For this reason, many women refuse to wear Chinese-made jewelry.
RED BRASS
Note that the basis for the jewelry of Czech companies was the tombak jewelry alloy (90% copper and about 10% zinc).
It is characterized by a high degree of resistance to corrosion processes, as well as increased ductility. It does not contain nickel, which is dangerous to the body. Typically, earrings are made with studs made of titanium, which is a medical material that does not cause allergies. All products manufactured in the Czech Republic are tested in accordance with Czech state standards.
Metals and alloys in jewelry
A precious metal is considered to be a rare element of high economic value. What is the reason for the value of precious metals? It's not just their rarity, but also their chemical properties: precious metals have a high luster, beautiful hues, are softer and more malleable, most melt well and alloy with other metals, and are subject to little or no corrosion. Of course, precious metals are used not only and not so much in the jewelry industry. Their properties determined the demand for them in various industries, and their rarity led to their use as money. Precious metals include gold, silver and the platinum group metals: ruthenium, rhodium, palladium, osmium, iridium and platinum itself. Platinum is much more common in trade than other platinoids, but they are also gaining popularity among jewelry connoisseurs who prefer new, interesting ideas. However, precious metals are not precious because they are used to make jewelry. There is another extremely rare precious metal called rhenium. It is not part of the platinum group or one of the traditional precious metals and is not used in jewelry. The value of precious metals may decrease due to the discovery of new deposits or improvements in methods of mining and refining ore. For example, after the discovery of aluminum, it began to be used as a precious metal, because... it was very expensive, more expensive than gold, due to the difficulty of obtaining it. But as soon as a cheap and easy way to obtain this metal was found, the price of aluminum gradually fell. Or silver, which was not mined in Ancient Egypt, but was brought from Asia and therefore was valued more than gold; in addition, silver was associated with the goddess Isis and endowed with magical properties. Gold Gold was one of the first metals that humanity mastered. It is known for certain that people have been familiar with gold for six thousand years, but, probably, their acquaintance with it occurred earlier: eight, or even nine thousand years ago. It was used by people almost all over the world to make money, jewelry, and works of art. The fact that gold is characterized by a native form led to such early acquaintance, although the total gold content in the earth's crust is very low. Pure gold is extremely rare and most of it contains from 2 to 20% impurities of silver, less often palladium, rhodium or copper. Natural alloys of gold and silver have two varieties: electrum, which has a greenish tint, with a silver content above 25%, and kustelite, containing from 10 to 25% gold and 90–75% silver. Pure gold is a very soft yellow metal, so much so that it can be scratched with a fingernail. This is why gold is always alloyed with other metals in jewelry to give it strength. Traditionally it is copper and silver. Copper gives gold a reddish hue; This kind of gold is called pink. White gold is an alloy of gold with other components: silver, platinum, palladium, nickel, which color it white. If, for example, one gram of ordinary 585-carat yellow gold contains 585 mg of pure 999.9-carat gold and an alloy (ligature) of silver and copper, then the white gold alloy contains nickel or palladium instead of copper. The more copper in the alloy, the redder the gold, the same effect from the presence of aluminum; silver, if more than half the alloy, makes gold pale yellow, green and white; cadmium gives gold its green color; steel gives gold gray, bluish-gray and even blue color; platinum is white; finally, palladium gives a beige or brown tint. The Russian measurement of gold purity in samples differs from the generally accepted standard in the world - the British karat system. One British carat is considered equal to one twenty-fourth of the weight of the alloy. 24-karat gold is pure gold without any impurities. For example, 18-karat gold means the alloy contains 18 parts gold and 6 parts impurities. In Russia, the sample indicates the number of weight parts of gold in 1000 parts of the alloy. 18K gold is our 750 purity gold. The purity of chemically pure gold corresponds to 999.9 fineness - it is also called “bank” gold, since bars are made from such gold. Gold of 999.99 purity is extremely expensive to obtain and is used only in chemistry. In the domestic jewelry industry, gold of 375, 500, 585, 750, 958, and 999 samples is used. Gold is one of the heaviest metals, only osmium, iridium and platinum are heavier: a ball of pure gold with a diameter of 46 mm weighs one kilogram. People have used this property throughout the history of gold: they washed away gold sand, which easily separated from the ground and settled. Lamb skins were used in this process, hence the origin of the myth of the Golden Fleece. Gold is completely non-corrosive - another advantage of using it in jewelry. To this day we can admire the products of Scythian goldsmiths. It seems as if these unsurpassed works of ancient jewelry were made just yesterday. Gold is very malleable and malleable. From a piece of gold weighing one gram you can draw a wire three kilometers long! For gilding, both electroplating and finishing with gold foil 500 times thinner than a human hair are used. Gilding methods were also known in antiquity: for example, in pre-Columbian America, but casting was not widespread in Europe; beating into shape was mainly used. All the objects of the famous Trojan treasure of Heinrich Schliemann, who passionately dreamed of finding the treasure of King Priam, were made in this way. People's passion for gold is reflected in the myth of King Midas, who wished that everything he touched would turn into gold, and even in the Bible, in the parable of the Golden Calf. But King Midas died of hunger, the Golden Calf was overthrown, and Schliemann’s gold turned out to be almost a thousand years older than Priam’s Homeric treasure. For centuries, alchemists searched for the philosopher's stone, which could turn everything into gold, which they considered the “king of metals.” This is how gold is at all times: it insidiously lures and destroys, deceives and pretends. But without it it is already difficult to imagine modern jewelry: it is a traditional material for their manufacture, a symbol of prosperity and success, a means of investment. In Russia, Erofey Markov is considered the first gold miner, a monument to whom stands in the city of Berezovsky near Yekaterinburg. After the discovery of the Ural deposits in Russia, state gold mining began, reaching its peak in the 70s of the twentieth century with the beginning of development in Yakutia. Paracelsus also suggested that gold had medicinal properties. Now they treat lupus, tuberculosis, leprosy, and isotope particles are used to treat malignant tumors. Modern electronics is impossible without gold, just like the jewelry industry. Despite the emergence of many new materials, gold is always in high demand due to its universal beauty, durability and compatibility with any precious stones. It is ideal for decorating colored stones, both precious and semi-precious. Silver Silver, a malleable, ductile white metal, became known to mankind later than gold, but earlier than other metals, and all for the same reason: it is often found in its native form, and does not need to be smelted from ores. The oldest silver items known to us were discovered in Iran - these are buttons that are almost seven thousand years old. In Assyria, Babylon and Egypt, silver was considered a sacred symbol of the Moon. In the Middle Ages, silver and its compounds were used by alchemists in their experiments. In addition to jewelry, silver is widely used as money. At first these were rings, bars, ingots, wire, small stumps that were in circulation in Mesopotamia and the Nile Valley. The first minted coin was made before gold, and this happened two and a half thousand years ago under the Lydian king Croesus (the same one), and the Romans minted silver money in the 3rd century BC. In Rus', silver coins appeared under Prince Vladimir; On one side of them there was an image of the prince on the throne (“table”), and on the other there was the inscription: “Volodimir is on the table, and this is his silver.” During the time of Anna Ioannovna, Akinfiy Demidov secretly minted his coins. The paradox is that the silver content in Demidov money was higher, i.e. they were more expensive than state ones. Silver coins are now issued in the form of commemorative and proof coins. Silver conducts heat and current well, which is why it is in high demand in the electronics industry. Pure silver is a soft metal, so to make jewelry it is alloyed with other metals, most often copper, zinc, and less often with cadmium, nickel and aluminum. Silver, like gold, is very malleable: from one gram you can stretch a wire two kilometers long. The property of silver halides to decompose under the influence of light into metallic silver ensured the use of this metal in the manufacture of photographic and film films. And another remarkable property of silver – bactericidal properties – has been known to people for about five thousand years. The ancient Egyptians applied a plate of silver to wounds; the Persian king Cyrus transported water only in silver vessels, in which it remained drinkable for a long time; The medieval physician Theophrastus Guggenheim (Paracelsus) treated some diseases with silver nitrate - lapis (lapis is still used in medicine for cauterization). Silver can kill 650 different bacteria because... Silver ions can disrupt the enzyme systems of microorganisms, inhibiting their growth and reproduction. When the troops of Alexander the Great reached India and entered the jungle on the banks of the Indus, intestinal diseases quickly spread among them, which strangely did not affect the military leaders and officers. Why? It’s just that the soldiers had tin dishes, while the officers had silver. This was enough to disinfect water and food. The tradition of making silverware among people of the upper classes grew in Europe into the custom of collecting “family silver.” Every self-respecting noble family had silver dishes and cutlery - a symbol of respectability and prosperity. Count Orlov, a favorite of Catherine II, owned 3,275 silver objects, which cost two tons of silver. The healing properties of silver, long noticed by people, apparently became the basis of legends about the magical power contained in it. Everyone knows that vampires can be killed with a silver bullet, that the best amulets and talismans are made of silver, that silver nails driven into a coffin will prevent the dead from rising. In France, brides and grooms still wear a silver chain before their wedding to avoid witchcraft. Perhaps such customs have a more serious basis than just superstition. This is illustrated by the belief that silver jewelry foretells a serious illness for its owner. Indeed, silver darkens with prolonged contact with protein and the skin of an unhealthy person. Gradually, the silver product darkens, oxidizes, and in the modern jewelry industry, many silver products are plated with rhodium. This gives the silver a particularly bright shine and does not allow it to darken, but, unfortunately, all the beneficial properties of silver are lost. Therefore, for preventive medical purposes and as amulets, it is better to buy uncoated products. Silver goes well with any cut stones, especially transparent ones with a glassy sheen, such as diamonds, topazes, citrines, etc. Platinum Native platinum is also one of the metals known to mankind for a long time. But its deposits are extremely rare, so it was not known on all continents. And although platinum is a soft and malleable metal, similar in color to silver, its very high melting point has become a major obstacle to the processing of platinum into jewelry. Platinum deposits have long been known in South America in Colombia and in Africa in Ethiopia. Platinum was unknown in Europe until the 18th century, when in 1748 the Spanish mathematician and navigator A. de Ulloa brought to the Old World samples of native platinum found in Peru. The beginning of the history of platinum in Europe turned out to be criminal: platinum is easily alloyed with gold similar in density, and it is very difficult to detect an admixture of platinum in gold, which is what counterfeiters took advantage of. In Spain, a royal decree was issued, according to which possession of platinum was punishable by law and it had to be thrown into the sea in front of witnesses. The name "platinum" is derived from the Spanish. “plata” means “silver”, and is disparaging in meaning (“serebrishko”, “silver”). In the middle of the 18th century, the unknown qualities of platinum were discovered: the metal did not rust or corrode under the influence of gases and chemicals. In Germany, laboratory equipment was made from platinum, in France - crucibles for melting glass, in Spain - tableware and watch cases. In Russia, native platinum was first found at the beginning of the 19th century in the Urals, and the largest currently available platinum nugget, the “Ural Giant,” weighing 7 kg 860.5 g, was also found there. It is stored in the Diamond Fund of the Moscow Kremlin. In 1828, about 1.5 tons of platinum were mined. Some hunters in the Urals used platinum as shot. If only they knew that in the near future this metal will cost more than gold! Russia is the first country in the world to use platinum for minting precious coins. But only a hundred years later, in 1956, the New York Mercantile Exchange proposed trading in platinum. At the same time, a gradual rise in prices began. No wonder Louis XVI called platinum “the only real value in the world, worthy of monarchs.” Platinum and its alloys are widely used in jewelry production. Every year the global jewelry industry consumes about 50 tons of platinum. The lion's share falls on Japan and China. In Japan, it is customary to buy platinum and diamond engagement rings. World-famous jewelry brands such as Cartier and Tiffany frame magnificent diamonds in platinum, including Nadezhda and Koh-i-Noor. Another advantage of platinum jewelry is the metal content of 90-95% purity, which never happens in products made from other precious metals. Platinum jewelry is an indicator of the most discerning taste and demands on the quality of materials. The platinum group metals, or platinoids, include platinum, palladium, rhodium, iridium, ruthenium and osmium. Platinum group metals are rare; their content in the earth's crust is very low. Extraction is expensive, and on the world market their price is higher than the price of gold. Even 40-50 years ago, the production and consumption of this group of metals in the world amounted to several tens of tons, mainly platinum was mined, which was used mainly for the manufacture of jewelry. Nowadays, platinum group metals are not only used to make jewelry, but also used as a coating, for example, platinum or rhodium. In addition, the method of blackening products made of bronze, tin and other metals with platinum group metals is widely used. These are mainly figurines, luxury items and interior decorations.Palladium was discovered by the English chemist William Wollaston in 1803. It was named after the asteroid Pallas, discovered shortly before palladium, and the asteroid was named after the ancient Greek goddess Pallas Athena. Palladium is used in alloys used in jewelry, for example, to produce "white gold", even a small amount of palladium in the alloy can dramatically change the color of the gold to a silvery white. The most technologically advanced and attractive alloys of palladium and silver in jewelry have a purity of 500 and 850. Palladium is predicted to have a brilliant future in jewelry. In terms of beauty, strength, resistance to corrosion, and rarity, palladium is not inferior to platinum, but it is cheaper, in addition, it is the lightest metal. With rising prices for precious metals, this is an excellent alternative to gold, silver and platinum. In addition, of all the platinoids, palladium is the best machined. Working with it is a pleasure: malleable and plastic palladium easily takes any shape. But the main property, very important in new environmental conditions: palladium does not cause allergic reactions. He's just perfect! And although many big-name jewelry houses are still eyeing palladium, Cartier took a risk and released a series of luxury Santos Mysterieuse watches in a palladium case encrusted with diamonds. The model was immediately recognized as the most stylish and modern. There were only 100 copies in the series. Palladium and platinoids in general are incomparably more popular in Asia. The world's largest producer of palladium jewelry today is China.
Iridium means “rainbow” in Latin. The metal received this name because many of its salts are brightly colored. A dense, very hard, brittle, silvery-white metal of the platinum family, iridium is known in the jewelry industry as a hardening agent for platinum. It is also used in medicine in radiation therapy to treat various forms of cancer. The rare platinum group metal ruthenium is used as a catalyst in some platinum alloys. Ruthenium was discovered and isolated by Russian scientist Karl Klos in 1844. Karl Klos named the element after his homeland, as he was born in Tartu, Estonia, which was at that time part of the Russian Empire. Small amounts of ruthenium can increase the hardness of platinum and palladium. Titanium's corrosion resistance increases markedly with the addition of small amounts of ruthenium. Ruthenium is sometimes alloyed with gold in jewelry. Fountain pen nibs are often made from alloys containing ruthenium. The Parker 51 fountain pen, known since 1944, was equipped with a nib containing ruthenium (96.2%) and iridium (3.8%).
Rhodium is a rare, silver-white, hard, wear-resistant metal used in alloys with platinum and as a catalyst. Rhodium plating is widespread in jewelry: a layer of metal is applied to items made of white gold, platinum, and silver by galvanic means. This gives the product a bright metallic shine and a reflective surface.
Osmium is a white metal with a gray-blue tint, very refractory, heavy, hard and brittle; cannot be machined; used in alloys with platinum to give them hardness. CopperCopper is one of the first base metals mastered by mankind. The Chalcolithic, or Copper Age, was the transition period between the Stone and Bronze Ages, when people found pieces of native copper and mistook them for stones. Trying to hit them with other stones in order to process them, as always, our ancestors discovered that some stones are deformed and can be shaped. This is how cold forging was discovered. The popularity of copper was subsequently determined by its availability for extraction from ore and its low melting point. Nowadays, copper is actively used in technology, while in jewelry, alloys using copper are mainly used: bronze, brass, etc. In addition, in jewelry it is customary to use alloys of copper and gold to increase the strength of products. One can only regret that copper jewelry has been forgotten. Why? Copper, like silver, also has bactericidal properties. This has been known for a long time, and even in Ancient Egypt, copper vessels were used to store clean water, and in the Cheops pyramid, archaeologists discovered part of an ancient water supply system made of copper pipes, which is still in working order (in our time, copper pipes can also be installed). In 2008, after extensive research, the US Federal Environmental Protection Agency (US EPA) officially designated copper and several copper alloys as germicidal surface substances. On a copper surface of a cutting table, for example, E. coli dies within four hours, while on a steel surface it lives for a month. Copper is also effective against a strain of Staphylococcus aureus. After all, it’s not without reason that copper coins were applied to the cones stuffed in childhood! Once upon a time, hospital door handles were also made of copper, and during times of plague, people who worked with copper or wore copper jewelry did not get sick. Copper and copper jewelry help lower temperature, relieve pain, have a hemostatic effect, normalize blood pressure, activate metabolism, calm the nervous system and improve sleep. Gypsy copper monistas and head hoops protected their owners from diseases during nomadic life. Yes, and pectoral crosses have always been copper. Copper may yet have a triumphant return as a popular metal for jewelry making. Its pleasant and unusual color seems to contain the heat of fire, casting its reflections around, and the gems acquire a dark, mysterious shine.
Bronze is an alloy of copper with tin, as well as other elements: aluminum, silicon, beryllium, with the exception of zinc and nickel. History records countless weapons, tools and jewelry made from bronze from 3500 to 1200 BC. This time is called the Bronze Age. The name “bronze” comes from the name of the port city of Brundisium (now Brindisi in Italy), which received copper from various deposits into the Roman Empire. Cargoes of tin from the British Isles also arrived there. Local craftsmen noticed that the alloy of these metals had good qualities and mastered the production of bronze. The history of bronze is, for the most part, the history of weapons. At first, swords, helmets, and various small parts such as buckles and spearheads were cast from it, then in the Middle Ages, bronze was used to make cannons. Bronze was used as a material for jewelry throughout Eurasia; It was used to make bracelets, rings, neck jewelry, brooches, clasps, etc. Bronze was often decorated with semiprecious stones, glass, and enamel. Nowadays, it is mainly used to make interior items using gilding; Gems look good in such products: malachite, jasper, rhodonite, agate, etc. Some jewelers make bronze replicas of archaeological finds - jewelry; To put on such a thing means to join antiquity, to the origins of human culture. Often on bronze you can see a film of various shades from green to brown. This is patina. It is usually formed on the surface of products made of copper, bronze and brass as a result of metal corrosion under the influence of the natural environment or as a result of patination, that is, heating or treatment with oxidizing agents. Artificial patina is used to protect works of art from destruction, and is also used for decorative purposes. Patina was also used by artists of Ancient Rome as a “patina of antiquity.” Patination is also called painting with special paint with an “antique” effect on products made of alloys and materials that do not contain copper, for example, plaster, wood, etc. This simple process has replaced such decoration methods as gilding, silvering, blackening, bluing, and oxidation.
Brass is an alloy of copper and zinc, yellow in color, sometimes with the addition of other elements. Despite the fact that zinc was discovered only in the 16th century, brass was already known to the ancient Romans. They obtained it by alloying copper with zinc ore. In African countries, brass is traditionally used as a material for making vessels, dance and ritual masks. In the 19th century in Western Europe and Russia, brass was often used to counterfeit gold. It was believed that the monument to Minin and Pozharsky, built at the beginning of the 19th century on Red Square in Moscow, was made of bronze. But restoration work showed that the material for this creation by the sculptor I.M. Martos was not bronze, but brass. It is usually used when an inexpensive metal that is sufficiently resistant to corrosion is needed. Decorations for books (clasps, corners) and crosses were made from brass.
Cupronickel is an alloy of copper (up to 70%) and nickel, similar in color to silver, is well processed in cold and hot ways, and is resistant to corrosion in air and water. Thanks to these properties, cupronickel is well suited not only for the manufacture of jewelry, but also for the production of dishes, cutlery, and pocket watches. Cupronickel silver was named in honor of its two discoverers, the French Maillot and Chorier, although such an alloy was known in China back in the 8th century BC. e. like pakfong. In the 13th century, products made from “Chinese silver” were more expensive than silver ones; Once in Europe, cupronickel immediately became the object of close attention of alchemists. The Chinese strictly guarded the secret of making the alloy, and until the 18th century, the Europeans, no matter how hard they fought, were unable to obtain “Chinese silver.” The French succeeded in this in 1812. The peak of popularity of cupronickel came in the 30s of the 19th century, and it was in demand among the middle strata of the population. Nobles and wealthy people still preferred silver, considering cupronickel to be an imitation of silver. Compared to nickel silver, cupronickel was easy to process, more resistant to oxidation and corrosion, looked more elegant, it could be coated with a thin layer of silver or applied with a silver ornament or blackened. Cupronickel, processed using filigree technique, goes well with Ural semi-precious stones. Most modern silver coins also consist of cupronickel with the addition of manganese.
Nickel silver (from German neusilber - “new silver”) is an alloy of copper and nickel with the addition of zinc. The history of nickel silver is closely connected with the history of cupronickel. When, at the end of the 18th century, German chemists tried to uncover the secret of “Chinese silver,” they were able to obtain an alloy that they called nickel silver, that is, new silver. Externally, German nickel silver, like Chinese pakfong, was difficult to distinguish from real silver. Special varieties of it have also appeared. Thus, argentan - nickel silver with the addition of tin and iron, was used in jewelry, and argentin - nickel silver with a high zinc content, was widely used in mechanics. Nowadays, nickel silver is widely used for making medals and jewelry. Nickel silver cutlery must be plated with silver, otherwise food will have a metallic taste. AluminumThe history of the use of aluminum in jewelry is interesting. In the mid-19th century, this light and ductile metal was extremely popular and valued higher than gold, but quickly depreciated after the discovery of a simple method for its production. But at first it was considered an exclusive material, intended only for the manufacture of art objects; aluminum coexisted with emeralds, garnets, corals and diamonds. Gold was often counterfeited with an alloy of aluminum and copper, and aluminum itself is similar to silver. The Roman Emperor Tiberius was once afraid of this, when he executed a master who presented him with a cup made of an unknown metal, light and similar to silver. Today, aluminum is making a comeback in the jewelry industry. As before, expensive luxury jewelry is made from it, as well as costume jewelry. One of the reasons for its success is the good compatibility of jewelry aluminum with precious stones, and especially with diamonds. In 1900, when the fashion for aluminum had long passed, the French jeweler Leon Colon presented a diamond tiara in the shape of a dove feather, 15 cm high, at the Universal Exhibition in Paris. Numerous diamonds of this jewelry are set in aluminum. The artist wanted to achieve a special lightness of the head decoration. In addition, the color of aluminum, which is more matte and neutral than other white metals, allowed the sparkle of the diamonds to be better highlighted. In silver they acquire a yellowish color due to the strong shine of this metal. In the 20th century, steel and titanium appeared among the metals used in jewelry, which are considered “masculine” metals: cufflinks, bracelets, pendants for men, tie clips, rings and rings are made from them. Steel allows you to achieve an interesting decorative effect - black, on which silver patterns appear. Jewelry made of steel and titanium rarely uses any precious stones other than transparent ones. Often these are unisex jewelry intended for young people. The metals that surround us are not always noticeable; some of them can be harmful or, conversely, useful. But those that have the opportunity to show their beauty in jewelry and luxury goods have been delighting people for thousands of years, and the longer, the more interesting and deeper the acquaintance with them becomes. Now, perhaps, all metals have been mastered and tested as jewelry, and we have the rare opportunity to choose any of them, be it gold or copper, bronze or palladium; each of them is good in its own way in combination with different materials and for different purposes, and here you can only be guided by your own taste. References:1. Andryushchenko A.I. Guide to gold and silver craftsmanship. Nizhny Novgorod. Printing house of the Provincial Board, 1904 2. Novikov V.P., Pavlov V.S. Hand-made jewelry. 1991
Protective coatings
Galvanic baths
In the production of costume jewelry, galvanic baths are used. This coating is applied in special electrolytic baths with solutions of gold salts, while current is passed through. As a result of this electrochemical process, one metal is deposited on another.
These types of coatings have increased wear resistance, in contrast to foiling, spraying, and painting. This advantage is achieved through interatomic bonding. Only alloys of two metals can be stronger.
Galvanic coating is more durable and can give the decoration durability. For such coatings, copper is the most successful component, since the metal is not dangerous to the body. In terms of chemical and physical properties, this is the best option for coating precious metals. Galvanic coating has two more important advantages - aesthetics and cost-effectiveness.
Plating with precious metals gives the products the appearance of jewelry, adding value to them in terms of design. The presence of precious metals in such small quantities, according to the legislation of the Russian Federation, does not give the status of “jewelry”, therefore they are not subject to special accounting and rules of circulation in the Russian Federation.
The percentage of gold content is very small (less than 20 microns), which does not lead to an increase in price. That is, the buyer takes almost a piece of jewelry at the price of costume jewelry.
The galvanic layer can disappear only together with the metal. This means that if there is mechanical damage, a copper layer will appear. Scratches and chips are also not desirable.
How to distinguish galvanic coating from other types?
The very boundary between the copper and gold layers is almost impossible to distinguish. Even if you deliberately scratch the product, for example, with pliers, you will not see this edge with the naked eye, since the metals interpenetrate each other. The same cannot be said about spraying or foiling.
Types of coatings
- containing rhodium, palladium, osmium (in expensive Czech and Italian collections, and the Jenavi collection);
- containing ruthenium (Korean and Italian collections);
- containing nickel (mainly Chinese jewelry);
- containing copper, bronze and brass (Italy and Czech Republic).
Only expensive Jenavi collections and costume jewelry of Czech and Italian origin are characterized by the presence of a galvanic layer of gold or silver from 0.1 to 0.3 microns. Such things do not darken with everyday wear and do not react with other elements from the air.
Purpose of the product
You might be surprised, but jewelry is not just something that people put on their fingers, neck, arms and legs. This concept is much broader. Just take a look:
- Personal jewelry
is something you wear on your body. Most often they are talked about when they mean jewelry. - Toilet items
are decorations that complement costumes and have practical uses. For example, tie clips or cufflinks. - Accessories
- products for toilet items. This could be a watch bracelet or a cigarette case. - Sets
are groups of jewelry with the same artistic design. For example, a collection of jewelry consisting of a ring and earrings. You can get acquainted with the AQUAMARINE collections in a special section of our website. - Tableware items
are products with practical applications. For example, spoons, forks and cup holders.
Jewelry also includes watches and icons made of gold and silver. They can be inlaid with precious or semi-precious stones, which we will talk about in the fourth article in the series of materials.
Silver
Not susceptible to oxidation even at elevated temperatures. It is worth noting that a thin film can appear during oxidation by oxygen plasma or ozone under the influence of UV rays. At high humidity with the presence of sulfur in the air, a dark coating can form. That is, the darkening of silver products is natural.
It is impossible to prevent blackening. You just need to constantly take care of your silver product and follow the tips for storing it. For example, silver items should not be placed next to an egg. Such proximity will turn silver black.
How to clean silver-plated jewelry fittings
To clean silver-plated components, do not use abrasives. Only 925 sterling silver can be rubbed with force. But silver-plated fittings require more careful handling. Under mechanical stress, the silver plating layer can wear away and expose the base alloy.
To make silver-plated components shine again, there are several ways:
- Blackened silver-plated fittings should be rubbed with a cloth designed for polishing jewelry. You can use flannel or cloth.
- To clean the patina on antique silver fittings, you can use a mild soap solution, followed by rubbing dry with a soft cotton cloth.
- You should not soak all the elements or the product with the solution at once. Try it first on a small area on an inconspicuous side, in case the product is not suitable.
Keep an eye on your jewelry and try to keep it in order!