Silver, properties of the atom, chemical and physical properties.
Ag 47 Silver
107.8682(2) 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1
Silver is an element of the periodic system of chemical elements of D.I. Mendeleev with atomic number 47. It is located in the 11th group (according to the old classification - a secondary subgroup of the first group), the fifth period of the periodic system.
Silver atom and molecule. Silver formula. Structure of the silver atom
Silver price
Isotopes and modifications of silver
Properties of silver (table): temperature, density, pressure, etc.
Physical properties of silver
Chemical properties of silver. Interaction of silver. Chemical reactions with silver
Getting silver
Application of silver
Table of chemical elements D.I. Mendeleev
Mineral deposits in nature
Based on the calculations presented by scientists, 700 thousand tons of silver were extracted from the bowels of the planet. Among all the precious metals, this mineral is the most common in nature. Its highest concentration is found in clay shales. Silver is found in deposits:
- native gold;
- copper sulfides;
- coal (see Countries leading in coal production);
- polymetals;
- oil (see How to reduce the dependence of the ruble exchange rate on oil prices? Expert answer);
- quartz.
There are fifty silver minerals in nature, but in industrial production nuggets and only a few substances containing its ions are used. The primary production of silver is no more than 20% of the total amount; it is mainly obtained together with gold, copper, and lead. Deposits of this precious metal are found all over the world. The most significant deposits of the mineral have been identified in Latin and North America, Australia, Russia, and China. Silver was discovered in European countries such as Armenia, Poland, Germany, Romania, and Spain.
Silver atom and molecule. Silver formula. Structure of the silver atom:
Silver (lat. Argentum) is a chemical element of the periodic system of chemical elements of D.I. Mendeleev with the designation Ag and atomic number 47. It is located in the 11th group (according to the old classification - a secondary subgroup of the first group), the fifth period of the periodic system.
Silver is a metal. Belongs to the group of transition metals, as well as precious metals and platinum group metals.
Silver is represented by the symbol Ag.
As a simple substance , silver under normal conditions is a malleable, ductile metal of a silvery-white color.
The silver molecule is monatomic.
The chemical formula of silver is Ag.
The electronic configuration of the silver atom is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. The ionization potential (first electron) of the silver atom is 731 kJ/mol (7.576234(25) eV).
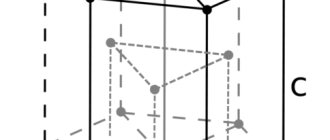
Structure of the silver atom. The silver atom consists of a positively charged nucleus (+47), around which 47 electrons move through five shells. In this case, 46 electrons are in the internal level, and 1 electron is in the external level. Since silver is located in the fifth period, there are only five shells. First, the inner shell is represented by the s-orbital. The second - the inner shell is represented by s- and p-orbitals. The third and fourth - inner shells are represented by s-, p- and d-orbitals. The fifth - outer shell is represented by the s-orbital. At the outer energy level of the silver atom, there is one unpaired electron in the s orbital. In turn, the nucleus of a silver atom consists of 47 protons and 61 neutrons. Silver belongs to the elements of the d-family.
The radius of the silver atom (calculated) is 165 pm.
The atomic mass silver atom is 107.8682(2) a. eat.
Silver , being a noble metal, is characterized by relatively low reactivity.
Sulfur, atomic properties, chemical and physical properties
History of mining and processing
Humanity has long noticed that silver looks great, is easy to process and does not corrode. Historians note its appearance in the form of jewelry and as a means of exchange no earlier than 4 thousand years BC. The most ancient developments of significant size were discovered in Cappadocia in the territory of modern Turkey. Metallurgy penetrated here as a component of the Kuro-Arakk culture from the Caucasus.
In nature, silver occurs in the form of nuggets, but most often it is found in a bound state as part of copper or lead ores. Man already by 2000 BC. e. established its extraction and processing. The goal was to make a crude lead-silver alloy, which was then cupellated (oxidative melting) to produce the metal in a relatively pure form. The most famous of the ancient mines were located at the Laurium mine in Greece. Active mining was carried out here from 500 BC. e. before 100 AD e.
Before the Renaissance, there were no special problems with this metal in Europe, Western Asia and North Africa, whose Berber tribes still wear exclusively silver jewelry. Not only coins, jewelry, but also household items were made from it. In those days, it was common practice to wrap a caftan around silver buttons and, if necessary, to pay off a creditor with them.
However, native silver began to be found less and less often, and the rich ores in the deposits dried up. Meanwhile, the need grew. The situation was saved by the unexpected discovery of the New World.
In the 16th century, silver mines were discovered by conquistadors in Mexico, Bolivia and Peru. Their further development led to an increase in the prosperity of the Spaniards and the recognition of South and Central America as the largest regions in the world for the extraction of this metal. But by the middle of the century, local reserves were depleted, costs increased and production began to decline.
Patio method
To better extract silver from ore, the Dominican theologian Bartolome do Medina invented the patio process in 1554. The design acquired its name for its similarity to an element of the classical architectural style. It is known that this method of obtaining precious metals was familiar to people back in antiquity, but then lost (now known as amalgamation). It involved mixing highly crushed ore with salt, water and mercury. The mixture was placed in a small space fenced off with a side.
For several weeks the components were heated in the open sun and mixed using mules. The complex reaction produced amalgam, mercury, and waste rock. This made it possible to eliminate the previously used smelting and significantly expand the extraction of precious metals in the New World. The technology retained its leading role until the 20s of the 20th century until it was replaced by a more progressive electrochemical method. Its main disadvantages:
- the presence of impurities in the final product;
- environmental pollution;
- harmful to the human body.
There is an opinion that American silver brought by the Spaniards to Europe provoked high inflation in the 16th and 17th centuries. There is no doubt about its role in the formation of a new economic formation, as well as in the development of trade relations with Asia and India.
In the middle of the 19th century. A large deposit of silver was discovered in Nevada. Thanks to this, the United States became the largest Ag producer in the world for several decades. Currently, Mexico, China, Peru, Australia and Russia are leading in terms of production.
Russian silver
It turned out that there were very few silver deposits on the territory of the Russian Plain. The demand for it was satisfied mainly by melting down foreign coins. Active searches for deposits begin in the 15th century. simultaneously with the formation of a centralized state and the growth of trade. By the beginning of the 18th century. The shortage of metals led to a decrease in the weight of coins, and budget needs required a doubling of the money supply.
Imported metal did not cover the needs of the economy, and Peter I, through laws and privileges, in every possible way stimulated the population to search for and develop deposits. The results of his efforts became noticeable in the first decades of the 18th century. By this time, the Nerchinsk deposits had produced 2 thousand kg of precious metal. Historical evidence of the importance of this work is a medal with the inscription “from home silver”, issued in 1721 in honor of the Peace of Nystadt.
Since then, more than 100 deposits have been discovered in the country. The most significant of them, Dukat, is located in the Magadan region. In addition to this region, the main mining areas in Russia include:
- Krasnoyarsk region;
- Chita region;
- Yakutia.
Characteristics by Zodiac signs
Silver is associated with the Moon, so astrologers recommend wearing metal products to the water signs of the zodiac Pisces, Cancer, and Scorpio. But this metal may not always be suitable for a person born under this element; there may be an allergy to it or the appearance of unpleasant sensations. The mineral has the most beneficial effect on people born under the influence of the planets Neptune, Venus, and the Moon.
It has an unfavorable effect on a person born under the influence of Mars, Jupiter, the Sun, mainly fire signs of the Zodiac.
Argentum, mineral of purity, purification, purity, inner strength. Talismans, amulets, amulet, and crosses are made from it, so anyone can wear this metal. The “heart” will tell you whether or not to wear silver jewelry; it depends on the desire of the person and the manufactured product that attracts him.
Application
Since ancient times, precious metal has served as a raw material for the manufacture of jewelry, weapons, coins, and dishes. The demand for the mineral has not decreased over the years; the scope of application of silver is constantly increasing; it is used in the production of:
- batteries and contacts in electrical engineering;
- chemical compounds;
- pharmaceuticals;
- instruments and equipment;
- submarines and nuclear boats;
- jewelry.
The metal is used in the food industry and to clean up oil spills. Nanoparticles obtained from the mineral are used to produce sensors that detect harmful bacteria.
In medicine
Even in ancient times, people treated diseases with metal, disinfected wounds and water with it. At the beginning of the last century, a suspension was prepared from colloidal silver, which was prescribed for therapy:
- colds;
- conjunctivitis;
- gonorrhea;
- epilepsy.
A mask with built-in microelement particles is worn by medical workers; it perfectly helps cope with bacteria. Protargol continues to be used as an antiseptic. Silver ions purify water from infection. The substance is used in pediatric dentistry in the treatment of caries and healing of pustular wounds.
Metal cleaning
- The ore is placed in a tank and treated with zinc dust. As a result, all impurities settle to the bottom, while the target raw material remains at the top. Subsequent cleaning is carried out in factories.
- The pyrometallurgical method produces pure silver. Copper or lead should be used as concentrators. These substances are treated with zinc at a temperature of 450 degrees - this way the metal particles dissolve better. After extracting the frozen substance, the precious metal is separated from it.
- At 1250 degrees, the zinc evaporates, but the remaining silver still contains arsenic and lead. For cleaning, oxygen treatment and heat exposure of 1000 degrees are used. This method is the most profitable, since lead and copper are mined in large quantities. Recycling such elements does not require significant costs.
- The purified precious metal is melted into ingots. No technology can guarantee 100% pure silver. To completely get rid of impurities, the ingots are transported to metallurgical plants for further processing.
If copper or lead is used as the main raw material, a pyrometallurgical purification technique is used. This method is advantageous because these impurities are inexpensive. As a result, the cost of the production process decreases, and the costs of producing less expensive metals are recouped. Electrochemical purification technology is used to separate silver.