Physical properties
Optical
- The color is white, due to impurities it can be different shades of yellow, greenish, bluish, red, colorless in small scales.
- The line is white.
- The shine of the units is matte.
- The sheen in solid masses is matte. Thin scales have a pearlescent tint.
- Transparency. In a piece it is opaque, but individual leaves are transparent.
Refractive indices
Ng = , Nm = and Np =
Mechanical
- Hardness 1.
- Density. 2.58—2.60.
- Cleavage is perfect, but macroscopically not determined.
- Kink. Shellish, earthy.
Chemical properties
Behavior in acids. Decomposes into H2SO4.
Other properties: greasy to the touch, when dry it easily absorbs moisture (sticks to the tongue), when wet it forms a plastic mass.
Diagnostic signs
Similar minerals are montmorillonite, sericite.
Identified by its clay-like appearance, softness, and oiliness to the touch. Similar to dense earthy calcite, but does not react with hydrochloric acid. To distinguish it from other clay minerals, special diagnostic methods are needed.
Associated minerals. Feldspars, opal, limonite. feldspathoids, muscovite, quartz, zircon, cassiterite, etc.; For the most part, they are found as relics in the kaolinite mass.
What is kaolinite and kaolin
Kaolinite is a mineral containing feldspar. When rock is processed, white clay (kaolin) is obtained.
Description of clay rock:
| Kaolinite | Kaolin (raw) |
| Clayey loose stone with a Mohs hardness of 2–2.5 points | A dusty rock that is white, gray, cream, or other light colored |
| The color is white, with a transition to yellow, cream, green, blue or red. There are brown spots | The deposit consists of a solid massif |
| The shine is dull, with a pearlescent tint | When moistened, it becomes slimy, plastic, soft |
| Opaque, thin plates show through under directional lighting | Splinters or rubs with slight pressure or impact |
| The break is uneven | May include sand grains or carbonate particles |
The name “kaolin” comes from the name of the territory in China - Gaoling District (High Mountain). Kaolinite continues to be mined here.
There are large kaolin deposits in the territories of Ukraine and Russia. The famous quarries are the Zhuravliny Log and the Kyshtymskoye deposits.
See the review of the mineral:
Application
It is used in porcelain and earthenware (together with feldspar and quartz), chemical (as a refractory), textile, paper, electrical insulation and paint industries, and in construction.
Kaolin wool is used as a thermal insulation material in various furnaces, gas turbines, furnaces, steam boilers, combustion chambers, and superheated steam pipelines. It is used to make sealing electrical insulating gaskets and filters for hot and chemically aggressive gases and liquids.
Usage history
The Chinese are the discoverers of kaolinite. It was these people who in ancient times learned to make porcelain from this clay of the highest quality. In the 3rd century BC. They created the Koalin "Terracotta Army", which numbered 8 thousand warriors on horseback.
In Russia and Western European countries, the secrets of producing porcelain from kaolinite were mastered only in the 18th century. The first factories were built in France and Germany. In Russia, a porcelain factory, built by order of the Emperor in St. Petersburg in 1744, still operates to this day.
Kaolinite
KAOLINITE (a. kaolinite; n. Kaolinit; f. kaolinite; i. caolinita, kaolinita) is a mineral of the subclass of layered silicates, Al4 Si4O10(OH)8. One of the main clay minerals. Polytype modifications of kaolinite are known: dickite and nacrite. Isomorphic impurities of Fe3+, Cr (up to 2.7% in chromium-K.), Ti (tenths), Fe, Mg, Ca, Na, K were established. Due to the high dispersion and layered structure, kaolinite is characterized by adsorbing impurities of oxides and hydroxides Fe, Al, Mn. Crystallizes in the triclinic system. The structure is based on a two-layer pack of tetrahedral silicon-oxygen and octahedral aluminum-hydroxy-oxygen (gibbsite) networks. Forms finely dispersed aggregates consisting of pseudohexagonal lamellar crystals up to 1 µm in size and less. Concretions, oolites, nodules, mealy and earthy loose accumulations are known. In its pure form it is white, sometimes with a bluish or brown tint. Mechanical impurities are colored red, black, bright green. The cleavage is very perfect along the pinacoid. Hardness 1-1.5. Density 2630 kg/m3. Kaolinite easily soaks in water, acquiring plasticity and disperses to form suspensions.
Kaolinite is a widespread secondary mineral. It is formed in weathering crusts during the hydrolysis of rock-forming aluminosilicate, mainly feldspar, rocks. It is part of various sedimentary rocks. Kaolinite is the main component of many clays (kaolin), sometimes forming large deposits. It is also the main component of individual zones in hydrothermally altered rocks, friction clays along tectonic faults; fills miarolitic voids in pegmatites. As the main component of kaolins, kaolinite is used for the production of paper (filler and coating), rubber, porcelain, earthenware, refractory materials, etc. Kaolinite is a potential source for the production of aluminum.
Processing schemes for kaolinite include wet or dry (air) classification, washing, disintegration (sometimes using liquid glass as a peptizer), wet or dry classification in spiral classifiers, hydrocyclones, air separators, and magnetic separation to remove iron minerals. From the leaching residues of copper-pyrite ore enrichment tailings, kaolinite can be extracted by flotation with a cationic collector and an alcohol foaming agent.
Origin, composition and properties of kaolinite
Kaolinite is a naturally occurring clay mineral formed during the weathering of feldspar-containing rocks such as granites and gneisses. Under the influence of water, weathering products first form sediment, then become compacted, acquiring a clayey consistency, and at the end of the process they are compressed into conglomerates, reminiscent of limestone in structure. As a result, an opaque, loose rock that absorbs moisture well is formed. The stone has a low density and a scaly structure. When wet, the upper scales become translucent, but after drying they become dull again.
The chemical formula of the mineral includes three main components: aluminum oxide, silicon dioxide and water. But often the mineral contains impurities: iron, manganese, copper, zinc, titanium, potassium, calcium. They give kaolinite various shades of red, yellow, green, blue or lilac.
Depending on the chemical composition and color, there are several varieties of kaolinite: rhodalite, a pink-colored mineral; lilac-green keffekelite; blue or purple teratolite; green chalk.
The hardness of kaolinite on the Mohs scale is 2.5, density – 2.6 g/cm³. During heat treatment, the mineral loses water, its molecular structure changes, and its density increases. This property is used in the process of producing porcelain. More than 15% of all rocks used in industry contain kaolinite. The mineral is also found in some semi-precious stones, thereby reducing their quality. For example, chrysocolla and turquoise, which contain kaolinite impurities, become more fragile and lose their brightness.
Kaolinite is a clayey natural mineral formed during the weathering process of feldspar-containing rocks such as granites and gneisses. We
also recommend reading:
The history and curse of the Kohinoor stone The marvelous ametrine stone Characteristics of larimar and its main properties Selection of stones by date of birth
Mining kaolinite is not difficult. The breed is widespread in nature: most areas of the planet have suitable conditions for its formation. Since the mineral has low hardness and density, it is easily extracted from the depths. Being a sedimentary rock, kaolinite lies almost on the surface: the mineral is mined only by open-pit mining.
Many countries near and far abroad specialize in the extraction of the mineral kaolinite. In Russia it is mined in the Urals, Eastern Siberia and the Far East; Large rock deposits are being developed in Ukraine and Kazakhstan. Among the non-CIS countries, Germany, Great Britain, France, Czech Republic, Spain, Portugal, USA, China, Angola, and Kenya stand out.
Kaolinite subgroup
This includes three polymorphic monoclinic modifications of the same substance, which from a chemical point of view can be considered as a basic aluminum silicate.
Kaolinite
-Al4[Si4O10][OH]8, or Al2O3•2SiO2•2H2O. The ancient name of this mineral came from the Chinese. “Kowling” is a high mountain (that’s the name of the kaolin deposit). Among the minerals of this subgroup, it is predominant. It is the main component of most clays.
Chemical composition
. Al2O3 39.5%, SiO2 46.5%, H2O 14%. The contents of individual components fluctuate somewhat. The following impurities are found in small quantities: Fe2O3, MgO, CaO, Na2O, K2O, BaO, SiO2, etc.
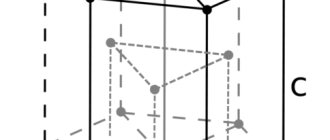
Rice. 340. Diagram of the crystal lattice of kaolinite (B) in comparison with the lattices of muscovite (A) and antigorite (B)
singonia
monoclinic;
monoclinic-domatic c. With. Crystal structure
. As in other mica-like minerals, tetrahedral silicon-oxygen groups (aluminum is not involved in them) are connected by three corners into a layer of an ordinary hexagonal network. Every fourth tip, occupied by oxygen, takes part in the structure of the lower “hydrargillite” layer (Fig. 340). In such bilayer packets, the total negative charge of the complex anion and hydroxyl anions is compensated almost completely by the positive charge of the Al cations. As shown in Fig. 341, at the contact of each layered package with the next one, hydroxyl groups are located on one side, and oxygen ions of the next tetrahedral layer are located on the other. This structure of kaolinite crystals simply explains the very perfect basal cleavage and easy splitting into thin plates of minerals of this group.
Rice. 341. Location and number of ions in individual sheets of the kaolinite lattice
Crystal Appearance
.
More or less well-formed plate-like crystals are extremely rare and small in size (up to 1 mm). It is very likely that they did not refer to kaolin, but to dickite. More often, fragments of curved columnar crystalline formations are observed, resembling earthworms in appearance (Fig. 342). Individual scales and plates have a hexagonal, less often rhombic or trigonal appearance. Aggregates
are loose, scaly or dense, fine-grained: sometimes found in the form of sintered forms.
Rice. 342. Fragments of worm-shaped kaolinite crystals. Magnified 20 times
Color
.
Individual scales and plates are colorless. Solid masses are white, often with a yellow, brownish, reddish, sometimes greenish or bluish tint. The luster
of individual flakes and plates is pearlescent, while that of continuous masses is matte Ng = 1.566, Nm = 1.561 and Np = 1.560.
Hardness
about 1. Individual scales are flexible, but do not have elasticity.
When dry, earthy masses appear thin to the touch. The cleavage
is very perfect along (001}. Cleavage directions are also established, parallel to the six-ray impact figures.
Specific weight
2.58-2.60.
Diagnostic signs
. Kaolinite in solid earthy masses is easily rubbed between fingers, when dry it greedily absorbs water, and when wet it produces an unusually plastic dough. Fine-crystalline differences in the corresponding preparations are recognized under a microscope by the shape of the scales and optical constants. Cryptocrystalline masses can be approximately determined by refractive indices, and more accurately by X-ray data, heating curves, and other methods.
P. p. tr. doesn't melt. HCl and HNO3 have almost no effect. In H2SO4, especially when heated, it decomposes relatively easily. Heated to a temperature of 500°, completely decomposes in HCl. White varieties, free from iron hydroxides, after calcination with cobalt nitrate take on a beautiful blue color (presence of Al).
Origin
.
In the main mass it is formed under weathering
of igneous and metamorphic rocks rich in aluminosilicates (feldspars, micas, zeolites): granites, gneisses, quartz porphyries, etc. This process of kaolinite formation occurs under the influence of H2O and CO2.
In this case, the alkalis, together with part of the SiO2 and alkaline earths in the form of carbonates, are carried away, and quartz and other chemically resistant minerals remain as inclusions in the clay mass, called kaolin
or kaolin clay. Often, masses of kaolin accumulated in this way are subject to erosion and are redeposited far from the place of their formation in stagnant basins in the form of layers of finely dispersed silty sediments, freed from coarse inclusions of foreign minerals.
Kaolinization phenomena also occur under low-temperature hydrothermal
processes under the influence, obviously, of acidic waters containing mainly CO2 on the same aluminosilicates and aluminum silicates that do not contain alkalis. This process essentially leads to the formation of pseudomorphs of kaolinite along certain minerals with the preservation of their external forms or outlines. These are, for example, pseudomorphs of kaolinite over feldspars, muscovite, topaz, scapolites, leucite, andalusite, pyrophyllite, etc.
During processes of regional metamorphism under conditions of high temperatures, clays transform into dense clay shales (mudstones and phyllites). Above 300°, kaolinite is completely destroyed, transforming in the presence of alkalis into sericite, mica, feldspars, etc., and in their absence, into aluminum silicates: andalusite, sillimanite, kyanite, garnets and other minerals that make up crystalline schists.
Practical significance
. Kaolin is used in many industries. Depending on the amount of foreign impurities, it is used either in its raw form, that is, without preliminary enrichment, or after elutriation in special installations.
The main and oldest consumer is ceramic
industry. Kaolin, free from iron oxide impurities, is used mainly in fine ceramics in the production of porcelain and earthenware. For this purpose, plastic properties are used, the ability to form stable suspensions with water, and most importantly, the ability to transform as a result of firing into a hard, durable stone-like material that does not soak in water and is stable at low and elevated temperatures. Refractory clays with a high melting point (not lower than 1580°), often containing hydrates of free alumina, are mainly used in metallurgy in the form of fireclay bricks, plugs, tubes, funnels, etc. For the manufacture of clay pots, tiles, pipes, etc. Low-grade sintering kaolin clays, called terracotta, tile, brick and others, are used.
In construction
In fact, clays as a water-retaining material are used as a protective layer under basement floors, for packing around foundations, in the construction of reservoir dams, in the production of adobe bricks, for the production of clay cement, etc.
On paper
In industry, kaolin is used as a filler and finishing agent to give paper a smoother surface, increased density, etc. In some types of paper, the kaolin content reaches 40%.
In other industries, kaolinite masses are used in the manufacture of oilcloths, linoleum, mixtures with drying oil and other substances, pencils, paints, in particular ultramarine (mixed with silica, soda, sulfur and organic substances), for the production of aluminum oxide, etc. It should also mention that in the form of so-called clay solutions (stable suspensions), fine clays are used when drilling exploratory wells for oil, salt and a number of loose minerals for the purpose of silting (filling small voids in fractured side rocks) and thereby preventing collapse of well walls, as well as for easier extraction of crushed ore fragments together with drilling fluid (thanks to the clay filler, the specific gravity of the fluid increases).
Place of Birth
.
Of the very numerous deposits, we will mention only a few. A large number of kaolin deposits are distributed on the territory of Ukraine
, in the weathering zones of outcrops of massive crystalline rocks of the South Russian Shield (granites, gneisses, syenites, etc.).
The most important of them are Glukhovetskoe, Turbovskoe and Raikovskoe (Vinnitsa region), Belaya Balka and Chasov-Yarskoe (Stalin region), etc. In the Urals
, a large number of primary and secondary deposits, mainly refractory kaolins, are distributed mainly along the eastern slope in Sverdlovsk and Chelya -Binsk regions: Kuryinskoye, Troitsko-Bainovskoye, Elenovskoye, etc. Refractory clays associated with lake and swamp carbonaceous sediments are also common in the Moscow region coal basin.
Among foreign deposits, we point out the largest deposits of primary kaolin in Cornwall and Devonshire
(England);
near Karlovy Vary
(Czechoslovakia);
in a number of places in Bavaria and Saxony
(Saxon and Bavarian porcelain);
near Limoges
in France (Sèvres and Limoges porcelain);
Kaolin of particularly high quality is known in China
on Mount Kau-Ling, near Yauchau-Fu and in other places.
Dickit
— Al4[Si4O10][OH]8.
system
. Before X-ray studies, it belonged to kaolinite. Named after Dick, who described the first discovery of this mineral under the name kaolinite in 1888.
Rice. 343. Dickite crystals. Increased by 200 times
The crystal structure is similar to kaolinite. There are the same continuous layers of hexagonal ring structure, but, unlike kaolinite, in each overlying layer the hexagons have a slightly different orientation. Dickite is often found in more or less well-formed transparent lamellar crystals of hexagonal shape up to tenths of a millimeter in diameter (Fig. 343). The ability of dickite to crystallize better is explained by the more symmetrical arrangement of OH ions in its lattice relative to O ions than in the structure of kaolinite.
Colorless, white in powdery masses, sometimes with a brownish, yellowish or greenish tint. Shine
pearl.
Ng = 1.566, Nm = I.562 and Np = 1.560. Hardness
is about 1.
Cleavage
is perfect according to {001}.
Ud.
weight 2.589 (theoretical). Dehydrates at 540°. It is most often found as a low-temperature hydrothermal mineral, often in association with sulfides, dolomite, fluorite, etc., in the form of crystalline crusts in drusy voids. It was also observed in chalcedony geodes.
In our country, dickite was found in significant masses in Central Kazakhstan, namely in Kara-Cheku (150 km south of Karkaralinsk) in the area of hydrothermally altered lava- and tuff-breccia porphyries. Pure dickite (mica) viscous rock is found here. Interestingly, the formation of dickite occurs following the sericitization of feldspars. The size of dickite scales reaches 0.1 mm, and in solid rock it is 0.5 mm.
Nakrit
— Al4[Si4O10][OH]8. Monoclinic system. The crystal structure differs from the structure of kaolinite by the smaller displacement of the layered packets relative to each other. It is found in larger lamellar crystals (up to 5 mm in diameter) of a hexagonal shape. It is also observed in radial lamellar aggregates and in dense or finely scaly masses.
Colorless or white with a yellowish tint. Shine
pearl.
Ng=1.563, Nm= 1.562 and Np= 1.557. Hardness
is about 2.
Cleavage
is very perfect according to {001}.
Ud.
weight 2.627.
Nacrite is formed both under exogenous and endogenous conditions, apparently in acidic environments. It is found in a number of deposits in the Ore Mountains (Saxony), in particular in Brand in the form of radially located groups of lamellar crystals around galena; in San Peters House in Colorado (USA) with mica and cryolite.