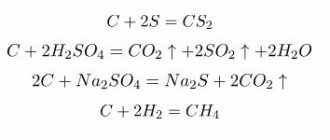origin of name
The Latin name for the mineral is galena - lead ore - mentioned by Pliny. The name galena was given by Kobel (1838).
Synonyms of the mineral galena:
Lead shine (Glocker, 1847). Apparently, mixtures of galena with other minerals include steinmannite (Ziepe, 1833), targionite (Bahey, 1852), cuproplumbite (Breithaupt, 1844), johnstonite (Haidinger, 1845), alyzonite (Field, 1859), furietite (Maine, 1860). ), guascolite (Dana, 1868), plumbocuprite and nolascite (Adam, 1869), paracobellite (Shrauf, 1871), kilmakuite (Tischborn, 1885). Fine-grained masses of galena with a matte sheen are called svinchak. Quirogite from the Sierra Almagrera (Spain), described by Navaro (1895) as a tetragonal mineral, according to X-ray data turned out to be galena of a special, distorted appearance. Castillite turned out to be a mixture of galena with Zn and Ag sulfides (Ramelsberg, 1866; Kalb, 1923). Richmondite (Skey, 1877) is a mixture of galena, fahlore, sphalerite and others. Plumbomangite (Köchlin, 1911) is a mixture of galena with various ore minerals.
An intergrowth of crystals with forms of uneven development on the faces and edges of a cube. Primorye Dalnegorskoye field
Use of mineral in magic
The lead luster and deep color of galena are external manifestations of its esoteric properties that attract magicians. Endowing its owner with spiritual strength and endurance, it helps to endure difficult trials with honor and easily cope with daily problems. A special property of the stone is the ability to transform negative emotions from failures into a creative direction for new achievements.
The crystal is a stone of success, giving its owners fame and recognition. It harmonizes a person’s thoughts, eliminating the risk of simple satisfaction with one’s success developing into complacency and pride. The crystal is recommended for people in creative professions, especially actors, whom the stone gives them expressiveness and the ability to analyze the world around them.
On topic: Mineral wulfenite: origin and features
It must be remembered that galena is a contact stone. The use of its magical properties is possible only when wearing jewelry or placing an amulet close to it. It is necessary to place the stone near your sleeping or working place and use it in meditation.
Chemical composition of galena
Theoretical chemical composition of the mineral: Pb - 86.60; S - 13.40. S is isomorphically replaced by Se; There is a continuous isomorphic series galena (PbS) - clausthalite (PbSe), various representatives of which are found in nature. Galena often contains an admixture of Ag, usually up to 0.1%, less often 0.5-1% and higher, which is partly due to the presence of inclusions of silver sulfides, partly due to the presence of AgBiS2 in the solid solution. Analyzes also show the presence of Zn, Cd, Sb, Bi, Cu, Sn, In, Tl, Au, Pt and others (usually no more than tenths of a percent); in most cases this is due to the presence of impurities of other minerals: sphalerite, boulangerite, chalcopyrite and others. Lead in galenas is a mixture of isotopes Pb2O4, Pb2O6, Pb2O7, and Pb2O8. The last three isotopes continuously accumulate in the earth's crust due to the radioactive decay of U and Th. The lead isotopic composition of galena can be used to determine absolute age.
Galena
Branches of application of galena
Galenas are practically not used in jewelry, this is due to the high fragility of the stone and significant difficulty in processing.
The exception is beautiful small crystals of regular cubic shape. Frames are made for them from precious metals, resulting in quite interesting jewelry and collectibles. Among collectors, samples of galenas, which are intergrowths of this mineral with other crystals, or high-purity galena crystals, are in demand. In addition, galena is the main ore for producing lead, as well as other metals that it contains as impurities, such as copper, silver, cadmium.
Varieties
Selenium galena from Rudny Altai, containing a small amount of Se, and intermediate members of the galena-clausthalite series from Colorado (USA), in which the PbSe content varies from 6.5 to 93.7 mol. %. Altai selenium galena has a density of 7.2 - 7.5. The cleavage planes have a dull sheen, and tarnish is often from bronze-yellow to bluish-black. Unlike ordinary galena, it is less resistant to weathering. It is found in the Zyryanovsky and Chudak deposits in the form of veinlets among copper ores in association with chalcopyrite, pyrite, and tetrahedrite. Galena-claustalites of the Colorado Plateau (USA) form inclusions in sulfide veinlets of uranium-vanadium deposits.
Svinchak is a dense matte galena.
Jewelry industry and stone care
The use of galena in the jewelry industry is not widespread. The most beautiful crystals are still used to make jewelry, framing them with precious metals.
There are products made of galena in the form of table decorations. Despite the fact that this stone is difficult to process, many connoisseurs want to see it in their collections.
Products made from such stone do not require special care. Owners should remember that the mineral is quite brittle and easily splits upon impact. It is better to store such a stone in a separate box with upholstery. Wipe with a soft dry cloth.
View this post on Instagram
Publication from Alena Mishinskaya (@mandragora) January 9, 2021 at 11:00 PST
Crystallographic characteristics
Cubic crystal system, hexaoctahedral class
Crystal structure Cubic face-centered lattice with four molecules per unit cell.
The main forms: cubic, cuboctahedral, octahedral, rarely trioctahedral and hexoctahedral. Cubic skeletal crystals are known. There are often unevenly developed crystals, elongated, columnar, elongated, and also tabular along the edge of a cube or octahedron.
Form of being in nature
The appearance of crystals.
Galena, pyrite. Druse of crystals
Crystals - from small to large (several centimeters in size) - are rarely ingrown; in most cases they grow and form drusen and groups.
Twins of the mineral at (111) are common; twins of intergrowth and germination are most common, often tabular, sometimes polysynthetic; twin growths are observed along (441), along (311) and (331), causing oblique streaking on the faces of the cube; Twin growths are indicated according to (520) or (730) in galena from Ratibořice (Czech Republic), secondary deformation twins are indicated according to (322), (221), (771) and (411). Forms oriented intergrowths with cotunnite, phosgenite and anglesite, pyrite, chalcopyrite, bournonite, fahlores, pyrrhotite, arsenopyrite, pyromorphite.
Aggregates.
The most common are grains and granular aggregates, less often - dense masses of druses, sometimes - sinter aggregates; Crystals and crystalline skeletal formations are relatively common.
Magical properties of the stone
Many believe that the stone is endowed with quite strong energy, which it completely, without reserve, gives to the owner. It is able to endow its owner with spiritual strength, endurance, protect against adverse effects, and helps to endure all life’s difficulties with steadfastness.
It is believed that galena can transform the negative energy of the owner into positive, charging it with positive emotions for a long period. It is recommended to wear the stone for people who are constantly in the spotlight, as the mineral can attract fame, success and fame. At the same time, it will protect its owner from star fever. Systematizes and organizes a person’s thoughts, prompts philosophical thoughts. Reveals the ability to make quick decisions in difficult situations, helps to make the right choice and conduct self-analysis.
Rare specimen of galena
The stone is widely used to create talismans and amulets. It is believed that wearing a ring increases perseverance and perseverance, while at the same time its owner becomes calmer. If you often have to make important decisions, it is recommended to wear earrings with a stone. Beads or a pendant with galena will help you cope with any difficulties and problems. It is recommended to place it in the sleeping area or office.
The stone is quite sensitive, so you need to contact it twice a week, sending it positive energy. If treated carelessly, galena will stop helping its owner.
Physical properties Optical
- The color is lead-gray, somewhat lighter in fine-grained aggregates; galena with octahedral separation containing Bi is somewhat darker; sometimes a mottled discoloration is observed.
- The streak is greyish-black.
- The luster is metallic, strong on the cleavage planes; galena with octahedral separation has a slightly duller luster; dense varieties are often matte.
Perfect cleavage in three directions
Mechanical
- Hardness 2-3.
- Density 7.4—7.6
- The cleavage is perfect (along the cube), in three directions parallel to the faces of the cube (100).
Sometimes separation at (111) is observed, characteristic of galena with a high bismuth content, which is explained by inclusions of bismuthin located partly along (111) of galena (the result of the decomposition of a solid solution of bismuth sulfide in galena) or the existence of a solid solution of AgBiS2 in galena. When heated, the octahedral separation disappears and is replaced by cube cleavage.
The fracture of the mineral in dense masses is flat-shell-shaped, uneven; in galenas with octahedral separation, the fracture is finely stepped.
Interesting facts about galena:
Jewelry with galenas is used as talismans. For example, a ring with galena gives its owner stubbornness, perseverance, and at the same time imparts prudence and peace. Galena beads and pendants are an excellent help in solving even the most complex and confusing issues. If you doubt your abilities, it is recommended to wear earrings with galenas. In general, galena is more inclined to help people with humanitarian inclinations, and for artists it is a true patron.
Galena. Chemical properties
The mineral dissolves in HNO3, releasing S and lead sulfate; When HCl is added to a nitric acid solution, a white precipitate of PbCl2 precipitates, soluble in hot water. Galena also decomposes with hot or strong HCl. Solutions of NaCl and CaCl2 have an effect on the mineral, especially at elevated temperatures and pressures. In polished sections, HNO3 quickly turns black, HCl turns slightly brown, and FeCl3 produces an iridescent tarnish; not etched by KCN, KOH, HgCl2, (NH4)2S. The structural features of the aggregates are revealed by etching with HCl (1: 1 or 1: 5). Microchemically, Pb is determined with KCN on a thin section, S - by imprinting on silver bromide paper. Film reaction: with a saturated solution of J in 5% KJ, when boiled, the mineral turns yellow-green.
Other properties
The mineral conducts electricity. On the octahedral face of the mineral, the electrical conductivity is higher than on the cube face. Electrical conductivity increases with increasing temperature, but above 300° it drops sharply (Niggli). Detects either positive or negative photoelectric effect. Galenas with a positive photoelectric effect do not have detector properties; Galenas, which give a negative photoelectric effect, are good detectors. Diamagnetic
Behavior of a mineral when heated: Melting point. 1112°. At high temperatures (above 350°), PbS forms solid solutions with AgBiS2. Ramdor (1955) explains this for the high Ag content in galenas that do not contain microscopically detectable Ag-bearing minerals. It is characteristic that in these cases a noticeable, often equivalent amount of Bi is always present. Thus, in galenas formed at average and elevated temperatures, it can be assumed that matildite is present in a solid solution.
The stone is easily chipped in steps along three mutually perpendicular planes.
Obtaining galena
It is easily obtained in various ways (Dölter, 1925), for example, by the action of H2S on acidified HNO3 solutions of Pb salts (amorphous and crystalline PbS); during the interaction of chloride compounds Pb with dry H2S in a heated tube; during the decomposition of lead sulfate in an atmosphere of H2 or CO; when lead sulfate interacts with rotting organic matter in water, when pyrite or marcasite is heated with a PbCl2 solution.
Crystals of galena and sphalerite with calcite
Diagnostic signs
The mineral is easily identified by its color, luster, characteristic cleavage in the cube, low hardness and high density. In fine-crystalline masses it differs from similar antimonous and arsenic compounds in density, behavior under the blowpipe and chemical reactions. In polished sections, the possibility of mixing galena with other common white isotropic minerals is almost excluded, since it clearly differs from them in the main characteristics: reflectivity, color, hardness, and especially in chipping triangles. In small grains it can be mixed with altaite (PbTe), clausthalite (PbSe), but the first is significantly, and the second is only somewhat lighter and much softer.
Satellites. Its most typical companion among the hypogene minerals in various deposits is sphalerite - these are the so-called polymetallic ores. ; often accompanied by pyrite and chalcopyrite. The vein minerals in most hydrothermal deposits are quartz, barite, fluorite, and calcite.
How to identify a fake
Natural galena looks almost like tin, pyrite and similar minerals.
It is easy to distinguish by several characteristics:
- The metallic color and shine are well expressed.
- Doesn't leave a mark on paper. On ceramics it is gray-black.
- Upon impact, it crumbles into crystal-like cubes.
Jewelers or decorators are not interested in galena. Being able to distinguish an original from an imitation is important for collectors of mineralogical collections and those who buy stones for magical or medicinal purposes.
Origin and location
Galena is one of the most common sulfide minerals of hydrothermal deposits, formed at various temperatures and in various geological settings. Medium- and low-temperature hydrothermal deposits are of greatest industrial importance. In the form of small rare grains, the mineral is found in pegmatites of granitic and alkaline magma, as a rare mineral - in igneous rocks and volcanic secretions. Supergene galena is found in some sedimentary formations. Sometimes the mineral forms almost monomineral ores (as, for example, in the Zavodinskoye deposit in Rudny Altai), but is usually accompanied by other sulfides.
Where and how it is used
Galena has found use among industrialists and collectors of stone collections.
Industry
Lead sulfide is a source material for a number of production processes:
- Manufacturing of ceramic products.
- Material for devices operating on semiconductors, as well as photodetectors and infrared detectors.
- Nanotechnology.
For industry, this is the main component in the production of lead by roasting. The process involves the associated extraction of valuable metals (silver, platinum, gold, copper, etc.) from galena ore.
In turn, galena can be obtained from pure lead. For example, exposing plumbum to sulfuric acid in a hot tube.
Other areas
Galena is inexpensive and available to collectors of any financial income. Samples with other breeds are especially in demand.
Jewelry and other products are created individually. The mineral is difficult to process; this is a segment of hand-made craftsmen who work on individual orders. The jewelry frame is made of jewelry alloy, copper, and silver. This honor is given to galena, the appearance of which meets aesthetic needs and is combined with other stones.
Place of Birth
In deposits among skarns in contact zones of granitoids and sedimentary rocks (mainly limestones), galena forms impregnations and granular aggregates, sometimes contained in significant quantities, accompanied by skarn minerals, sphalerite, chalcopyrite, pyrrhotite, etc. Examples: Altyn-Topkan in the Karamazar Mountains (Tajikistan ), Tetyukhe (Primorsky Territory), Kyzyl-Espe, Aksoran and Akchagyl (Northern Balkhash region, Kazakhstan), Savinskoye and other deposits of the Chita region, Darwin (California, USA), Schwarzenberg (Saxony, Germany) and others. In lead-zinc ores that form deposits and veins, galena, in close association with sphalerite, is accompanied by pyrite, chalcopyrite, often arsenopyrite, as well as fahl ores, pyrargyrite, stephanite, bournonite, boulangerite and other complex sulfides containing Ag, Pb, Cu . Occasionally it is also accompanied by Ni sulfides and arsenides.
Typical hydrothermal, predominantly medium-temperature deposits are: lead-zinc deposits of the Rudny Altai (Kazakhstan and Altai Territory) - Ridderskoye, Zyryanovskoye, Zmeinogorskoye and others; Sadonskoe vein deposit (North Ossetia Russia); deposits of the Mekhmaninsky ore field (Azerbaijan); some deposits in the central part of Kazakhstan (Berkara, Maykain, Aleksandrovskoye, Kurgasyn, Azhim); Achisay, Mirgalimsay deposits; in the Karatau mountains (Kazakhstan); in the Chita region—fields of the Nerchinsky district (Troitskoye, Smirnovskoye, Kadainskoye); vein deposits of Pribram (Czech Republic), Freiberg and Clausthal (Germany), Coeur d'Allen in the state. Idaho; Leadville in Colorado (USA), Sullivan (Canada), Santa Eulalia (Mexico), Broken Hill and Mount Isa (Australia), Bodwin (Burma) and many others.
Galena, chalcopyrite. Crystal intergrowth
Relatively low-temperature deposits include the Bleiberg deposit (Austria), some deposits in Silesia (Poland), Raible (Northern Italy), and deposits of the “lead belt” of Missouri (USA).
In varying quantities, galena is also found in essentially copper deposits (in Russia - Dzhezkazgan, some pyrite deposits of the Urals), in deposits of the sulfide-cassiterite formation (Yakutia, Primorsky Krai), in iron ore deposits (Bakalskoye deposit, Chelyabinsk region), in gold ore deposits quartz veins (Berezovskoye deposit, Sverdlovsk region), in tungsten and molybdenum deposits (North Kounradskoye, East Kounradskoye, Karaobinskoye deposits in Kazakhstan).
Supergene galena in sedimentary rocks occurs as a result of reduction by organic substances from lead sulfate or from the action of hydrogen sulfide on solutions of lead salts. Forms crusts and deposits on pyrite and marcasite nodules, dissemination and thin films in coals (Borovichi district, Novgorod region). It is found in limestones of various ages, in paragenesis with pyrite and marcasite, in the form of grains and crystals. In the Triassic deposits of Mount B. Bogdo (Astrakhan region), galena was found as an interlayer in limestone. Occasionally observed in rocks of various Cambro-Silurian and Devonian horizons near St. Petersburg. In cuprous sandstones and sandy limestones of Cambrian age, galena is observed in the form of syngenetic dissemination (upper reaches of the Lena, Irkutsk region). In the ore-bearing variegated Triassic sandstones of the Kemern-Mechernich region (Germany), galena, together with cerussite, chalcopyrite and copper carbonates in the form of nodules and scattered dissemination in the sandstone, is confined to a specific layer.
Galena is relatively common in phosphorite nodules of Podolia (Ukraine); it forms regular cubic crystals or fills irregular cavities inside the nodules, and is also located in their outer parts along the rays of the phosphate substance.
As a modern new formation, galena is noted in old mines: together with sphalerite in the form of deposits on iron chains in the mines of Upper Silesia (Poland), on abandoned tools - in crystals up to 12 mm in size in Missouri (USA).
Galena pseudomorphs are known from cerussite, anglesite, pyromorphite, chalcocite, bournonite, fahlores and wood. The typomorphic properties of galena from hydrothermal deposits are to some extent the appearance of the crystals and the content of impurities. Crystals of high-temperature hydrothermal galenas are often cubic in appearance, while those of lower temperatures are often octahedral in shape; High-temperature galenas are often bismuth-bearing, while lower-temperature galenas usually contain Ag and Sb.
Galena, chalcopyrite, quartz
Where is the mineral mined?
The crystal has a very wide distribution halo; this stone can be found (naturally, with the help of specialists) almost everywhere. The most popular deposits are located in the following countries:
Russia;- USA;
- Kazakhstan;
- Italy;
- Poland;
- Mexico;
- Australia.
Mining this mineral is hazardous to health, as harmful dust settles in the lungs of workers and researchers. Therefore, when carrying out work, safety measures are strictly observed. The long process of lead extraction negatively affects all body systems. We can say that galena is a toxic and dangerous mineral.
But modern developments make it possible to minimize the chances of poisoning. There are barrier devices that protect against lead dust. Lead mining, processing and refining are complex and toxic procedures.
Physical research methods
Differential thermal analysis
Main lines on radiographs:
Ancient methods. Under a blowpipe on coal it cracks and shatters into pieces, but melts quietly in a fine powder. The coal near the sample is covered with a yellow coating of PbO with a bluish border (PbCO3). (Se - containing varieties form a reddish-brown coating on the coal near the sample with a narrow dark border; a characteristic, albeit weak, odor due to Se is detected). With soda on coal it produces a beetle of Pb, which after prolonged blowing either completely disappears or, if Ag is present, leaves a small beetle of Ag. Releases SO2 in an open tube
Crystal optical properties in thin preparations (sections)
Refractive indices 4.015 (C), 3.912 (D), 3.796 (F) (Winchel). In reflected light it is white and serves as a standard for white color. Reflectivity (in%): for green rays 43.5, for orange - 37.5, for red - 35; according to Folinsbee, measured using a photocell - 42.4. Isotropic. Sometimes it is anomalously anisotropic as a result of pressure or due to the presence of an isomorphic impurity α-AgBiS2. Galena is polished well in fine-grained aggregates, worse in coarse-grained ones. A characteristic diagnostic feature is the presence in polished sections of chipping triangles, identically oriented within single-crystal grains; the reason for their formation is perfect cleavage along the cube. Etching or weathering sometimes reveals a fine zonal structure, especially in low-temperature galenas.
Talismans and amulets
Any specimen of galena can serve as a powerful talisman for its owner. The gem carries the energy of metal and its main purpose is protection against witchcraft and magic, as well as karmic in nature.
This is interesting!
The ancient Greeks wore lead tablets on their chests to protect themselves from externally directed negativity. Their mythology records that Bellerophon, with the help of Athena, was able to saddle the winged horse Pegasus. He also defeated the terrible fire-breathing monster Chimera by throwing a piece of lead (galena) into its throat. The heat in the mouth melted the lead and burned through the insides of the monster.
Today, galena is used as a talisman for various purposes:
- A stone at the entrance to a house is used to scare away ill-wishers and envious people, as well as to protect the home from curses.
- A ring or ring with a galena insert (worn on the ring or middle finger) - for self-confidence, determination and steadfastness of will.
- Necklaces, beads and pendants - to quickly find the right solution.
- Any jewelry or rough stone as a protective amulet.