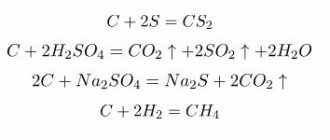Chemical composition
Double salt CaC03-MgCO3. The contents of CaO and MgO often fluctuate within small limits. Isomorphic impurities in addition to Mg: Fe2+, when dominant, leads to another type of dolomite group - ankerite, sometimes Mn2+ (from several percent to dominance, in kutnogorite), occasionally Zn, Ni and Co (in red dolomite from Příbram in the Czech Republic the content is Co[CO3 ] reached 7.5%). There are known cases of inclusions of bitumen and other foreign substances in dolomite crystals.
Varieties
Ankerite was named in honor of M. Anker (1772-1843), a mineralogist from Styria. It is a transition member to pure CaFe(CO3)2 or "ferrodolomite".
Kutnagorite (named after the locality of Kutnagora in Czechoslovakia) is essentially “mangandolomite.”
Cobalt (with CoO about 5%) and lead dolomites are also known. Continuous isomorphic series apparently exist between CaMg(CO3)2 and CaFe(CO3)2, on the one hand, and CaFe(CO3)2 and CaMn(CO3)2, on the other.
Crystallographic characteristics
Syngony. Trigonal.
The symmetry class is rhombohedral. With. L36C. Etc. gr. R3– (C 23i). a0 = 4.822; с0 = 16.11 (at the ratio Ca : Mg = 1 : 1.1). a = 102°50′, Z = 2 for a cell of a cleavage rhombohedron; ag^=6.01 A, a = 47°30't Z = 1 for the simplest unit cell; a0 = 4.84 A, c0 = 15.95 A, Z = 3 for a hexagonal cell.
Crystal structure
Coarse-crystalline dolomite with talc and quartz.
Eastern Sayan Mountains. Its structure is homeotypical of calcite; Characterized by the fact that calcium (Ca) and magnesium (Mg) ions alternate along the triple axis. The replacement of large calcium atoms with smaller magnesium atoms leads to a decrease in the symmetry of the crystal structure and morphology of dolomite.
Crystal shape. Its crystals are usually rhombohedral {1011}, sometimes with a predominance of the {4041} form in combination with {1120}, {0001} or with rhombohedrons of the second and third order. (1011) D (TlOl) = 73°16′, (0001) D (4041) = 75°16′. The faces of the rhombohedron {1011} are often curved or composed of subparallel individuals and take on a saddle shape.
Description of the stone
The rock got its name thanks to the name of the geologist from France D. Dolomier, who was the first to describe the composition and properties of the mineral. Limestones and dolomites belong to sedimentary rocks, and most often dolomite is part of limestone. The stone is formed during hydrothermal processes, which occur under high pressure at depths containing hot water solutions. The formation of the mineral depends on the presence of chemical precipitation and salts in sea and lake waters. Also, under the influence of water seeping through the limestone silt, limestone is replaced, resulting in the formation of dolomites.
Form of being in nature
The appearance of crystals. Frequently occurring crystals have a rhombohedral appearance. In contrast to calcite, rhombohedra {101–1} are widespread, and saddle-shaped curved faces and spherocrystals with curved cleavage surfaces are not uncommon.
Twins along {0001}, {1010}, {1120} and {0221} are common; the last type of twins is lamellar, found in mechanically deformed dolomite crystals. Typically, dolomite occurs in massive, granular, and dense aggregates as a rock-forming mineral.
The aggregates are usually crystalline granular, marble-like, often dense or porous, less often kidney-shaped, cellular, spherical, etc.
Dolomite utensils
They learned how to make dishes from this mineral. Thanks to natural material, the products are considered environmentally friendly and harmless. The glaze adheres flawlessly to dolomite, making the kitchenware very beautiful. Due to the fact that natural stone has a porous structure, the objects are very light in weight. The disadvantages are: the high fragility of the dishes and the inability to use hot dishes, since the high temperature causes the glaze on the surface of the product to become cracked.
Therefore, it is recommended to use dishes for cool drinks and fruits. You can also purchase dolomite items to use as kitchen decor.
Physical properties
Optical
- Color. Colorless or white ( dolomite ), sometimes grayish-white with a yellowish, brownish, or less often greenish tint. Light to dark brown (ankerite) or pinkish (kutnahorite).
- Trait. White, light gray
- Shine. Glass.
- Transparency. Translucent, transparent.
Refractive indices
Nm = 1.681–1.695 and Nр = 1.500. . Nm = 1.681–1.695 and Nр = 1.500–1.513. In cathode rays it glows bright orange-red. no = 1.679, ne = 1.500 (Na-light) for pure dolomite; refractive indices increase with higher iron content.
Mechanical
- Hardness 3.5 - 4. Brittle.
- Density. 2.8–2.95 for pure dolomite, increases to 3.2 for ankerite and kutnahorite
- Cleavage perfect according to {1011}.
- The fracture is granular, conchoidal.
Chemical properties
Behavior in acids. Reacts with hydrochloric acid in powder. In a pile with cold HCl it dissolves slowly, and in a heated pile it dissolves faster (with strong boiling); Calcite boils strongly already in cold HCl.
Diagnostic signs
Similar minerals. Calcite (lime spar), magnesite, chabazite.
Like other carbonates of the calcite series, dolomite is characterized by rhombohedral cleavage. Dolomite crystals in most cases also have a rhombohedral appearance. In individual grains, without chemical analysis and measurement of optical constants, it is impossible to distinguish from ankerite, sometimes siderite. It is very characteristic that dolomite, unlike calcite, exhibits polysynthetic twinning not along (011–2), but along (022–1). In transparent bodices, this direction of twinning is established along the short diagonal of the rhombuses formed by the cleavage cracks.
Diagnostics. Difficult to recognize. It reacts with hydrochloric acid only in powder, which is why it differs from similar calcite, magnesite and siderite.
Related minerals .
Pyrite, marcasite, hematite, realgar, tremolite, calcite, gypsum, anhydrite, halite, serpentine, talc.
Jewelry with mineral
As a rule, dolomite jewelry is original work, since there is no mass production of jewelry with this stone. But it is not used in jewelry because of its fragility and softness.
However, dolomite is actively and successfully used in the works of private craftsmen. Mostly dolomite jewelry is made in ethnic style. This means that they are not suitable for everyone. Such jewelry is preferred by people who love a similar style of clothing and lead a certain lifestyle. These are people of creative or free professions.
Necklace
Such necklaces are often made of leather, suede, and beads. But its main decoration is processed dolomite. Such things are exclusive (made in a single copy). That's why they are not cheap. For example, a designer necklace with dolomite costs from 1,500 to 5,000 rubles. And sometimes the price reaches up to 15,000. It all depends on the materials used.
Beads
This is a beautiful and unique decoration. In most cases, landscape dolomite is used to make beads; it has a color and pattern. Sometimes the patterns are so bizarre that they resemble landscapes or oriental paintings. No bead is alike.
And at the same time, they may have geometry flaws, natural roughness, chips and chips. Such flaws are not a disadvantage, but a highlight of natural stone.
A very popular color when making beads is called “Golden Autumn”, where yellow spots are scattered on a light gray background. Sometimes beads are sent with silver, copper or white gold. Such jewelry can cost from 3,000 to 20,000 rubles. The frame is of great importance here.
Also read: Petersite - a powerful mysterious stone
Earrings
For example, earrings with dolomite in a copper frame cost 1,000 rubles. The same earrings in a silver frame cost 6,000 rubles. And gold earrings with polished landscape dolomite cost from 10,000 rubles, depending on the amount of gold used and the size and number of stones. Unusual specimens of dolomite are also used in the manufacture of earrings.
Reference. Particularly popular is the stone with a “realistic depiction of the landscape.” Such a landscape on stone was painted by nature itself without human hands. It is difficult to obtain such specimens, since they are mainly found in private collections.
You can purchase or order such jewelry only from private craftsmen (authors) who work specifically with this natural material. By agreement, we can discuss the design, cost, and materials from which the product will be made.
Dolomite dishes
Dolomite dishes have several features that distinguish them from porcelain or earthenware:
- brighter;
- cheaper;
- more fragile;
- less heat resistant;
- lighter.
How to navigate when buying dolomite cookware:
- Dolomite dishes are lighter than porcelain or earthenware dishes. But at the same time it is also more fragile. Therefore, when purchasing, you should choose heavier products. They are stronger and will last longer. As a rule, the price of such products is slightly higher.
- When purchasing, you can lightly tap the product. If the sound is clear and booming, then the clay is fired evenly and such a product can be purchased. A dull sound indicates that the product is of poor quality or the manufacturing technology is broken.
- Suspiciously cheap. Products that are too cheap should raise red flags. Sometimes, instead of natural dolomite tableware, you can purchase a synthetic fake. In order to further reduce the price, natural material is replaced with synthetics. This product can be identified by its characteristic chemical smell. If such a smell is present, then this is a fake and you should not buy it.
Attention! Dolomite cookware does not tolerate high temperatures; you cannot pour boiling water into it, as this will lead to the formation of microcracks. Such products are only suitable for cold foods or for interior decoration.
The properties of dolomite, its price, and various colors make this stone a universal material. If previously dolomite was used exclusively in construction, now it is used in architecture, the chemical industry, medicine, and metallurgy.
Dolomite is perfect for making decorative items and jewelry. The stone has positive energy and has a beneficial effect on humans. Caring for it is easy, and the product looks original.
Also read: Scolecite - a stone of peace and harmony
Origin and location
Dolomite Khabarovsk Territory
Along with calcite, it is a widespread rock-forming mineral.
In typical vein hydrothermal deposits, it is much less common than calcite. When processing dolomitized limestones with hydrothermal solutions, coarse-crystalline masses of dolomite are often formed in association with magnesite, calcite, sulfides, quartz and other minerals (Tilkerode, Harz).
The main masses of dolomite are associated with sedimentary carbonate strata of all geological periods, but most of all Precambrian and Paleozoic age. Dolomites in these strata often form entire massifs or interlayer with limestones, and are sometimes observed in the form of not quite regular deposits, nests, etc.
The question of the details of their origin causes great debate. At present, dolomite is not deposited in marine basin environments, but in the geological past, in a number of cases, dolomites formed as primary sediments in aqueous salt-bearing basins, as indicated by their association with sediments of gypsum, anhydrite, and more soluble alkali salts.
In other cases, dolomitization of previously deposited sediments of calcium carbonate undoubtedly took place: facts of replacement of shells, corals and other calcareous organic remains with dolomite are observed.
In the weathering zone, dolomites slowly dissolve, collapse and turn into a loose fine-grained mass.
How to distinguish an original from a fake
As imitations, an artificial analogue of a mineral that has similar properties is usually used. Its advantages are that it is much cheaper and more varied in color. Therefore, it is perfect for those who want a beautiful decoration, and not a talisman.
Artificial dolomite does not have healing or magical properties!
Dolomite
It is almost impossible to distinguish natural dolomite from a fake with the naked eye. This can only be done in laboratory conditions using hydrochloric acid, to a drop of which the natural mineral reacts very reluctantly. The artificial stone in its place will immediately boil violently.
However, experts make several recommendations. For those who still decide to buy, you need to:
- Ask the seller to provide a certificate, which always indicates the origin of the mineral.
- Carefully examine the patterns on the sample. With natural stones they are always chaotic; with imitations, a certain pattern will certainly be revealed in the design.
- Smell the stone; artificial analogues are distinguished by the presence of a chemical “aroma”.
All these signs, except for the certificate, are conditional, but sometimes they help to identify deception.
Dolomite deposits
Dolomite deposits are widespread along the western and eastern slopes of the Urals, in the Donbass, on the banks of the Volga and in other places. The main mass of dolomites is confined to carbonate strata of Precambrian and Permian age. Of great interest are the modern processes of dolomite formation in the lake. Balkhash (Kazakhstan), recently studied in detail by N. M. Strakhov.
In Central Europe, dolomites are known in Zechstein. Dolomite quarries are found, for example, in Wünschendorf, in Kaschwitz near Gera, dolomite marbles are known in Krotendorf, Hammer-Unterwiesenthal, Oberscheib, Raschau, Hermsdorf and in other areas of the Ore Mountains.
Production
Dolomite is often found in hydrothermal deposits. It can also form when calcite is replaced under the influence of sea or groundwater.
Since dolomite is a sedimentary rock, its strength is determined by its depth. The deeper the mineral layer is, the stronger it is.
There are dolomite deposits in Switzerland, Spain, and the North American continent. The largest of them are in Mexico and the Lake Ontario area. It is also found in the Caucasus, in the Moscow region, in the Ural Mountains, and Central Asia.
Dolomite is extracted in quarries using the drilling and blasting method or using borehole charges. Its processing includes crushing, roasting and in some cases grinding. The material is crushed into pieces using hammer or jaw crushers. Dolomite is usually fired in shaft-type kilns with remote fireboxes. Ball or other mills are used for grinding.
This is how an explosion occurs in a dolomite quarry:
By firing at different temperatures, different materials are obtained. Caustic dolomite is fired at temperatures up to 750 degrees. At higher temperatures (up to 850 degrees), dolomite cement is formed. Dolomitic lime, capable of slaking, is produced at a temperature of about 950 degrees.
If the temperature rises to 1500 degrees, metallurgical dolomite is obtained, which is used to produce refractory materials. This material does not react with water and has no astringent properties.
Dolomite flour is made by crushing and finely grinding raw materials; it contains ordinary dolomite.
For its production the following equipment is required:
- crushing plant;
- mill or crusher for crushing rock;
- feeding vibration mechanism;
- vibrating sieve.
The technological process for producing dolomite flour includes primary and secondary crushing, grinding into small fractions, thorough drying and heat treatment. It is then packed into containers or bulk bags. Dolomite flour is stored in closed dry warehouses.
Grinding the material to flour: